Figures & data
Figure 2. Overlay of UPLC chromatogram at 280 nm top and base peak intensity (BPI) chromatograms (bottom) of BPFRF.
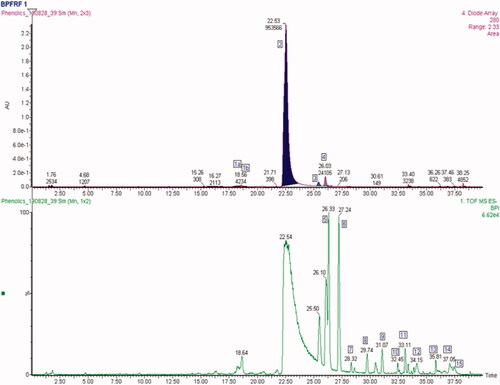
Figure 3. Photodiode array detector (PDA) spectra and mass spectra (MS/MS) fragmentation pattern present in B. pinnatum flavonoid-rich fraction (BPFRF). Luteolin C-glucoside-C-arabinoside (carlinoside) (A). Quercetin (B). Luteolin (C). Quercetin-3-methyl ether (isorhamnetin) (D). Luteolin-7-glucoside (E).
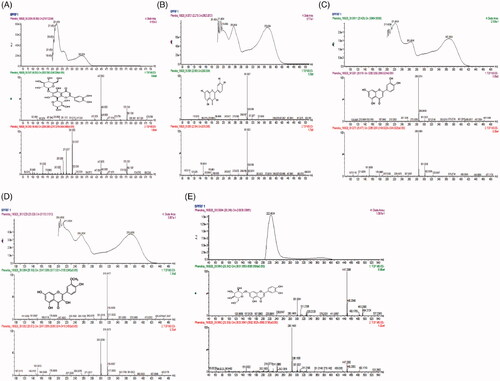
Table 1. Constituents of BPFRF identified and characterized by UPLC-PDA-Q/TOF-MS2 analysis.
Figure 4. 2,2-Diphenyl-1-picrylhydrazyl free radical scavenging effect of BPFRF in comparison with ascorbic acid (3.125–100 μg/mL).
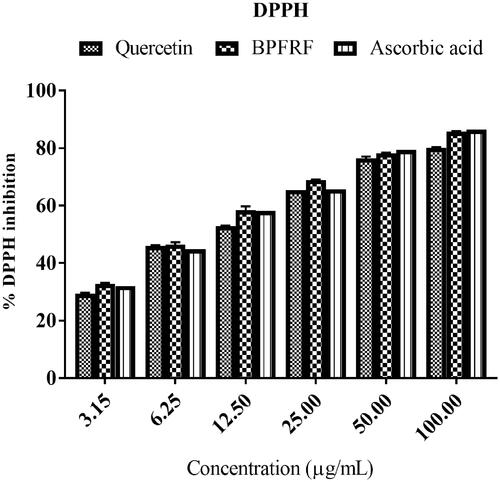
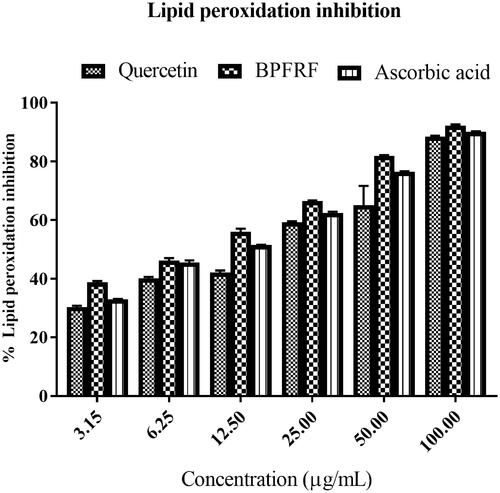
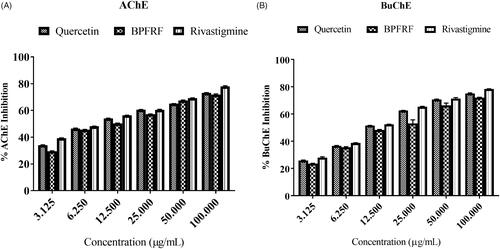
Table 2. DPPH radical scavenging potential, lipid peroxidation inhibitory activity and cholinesterase inhibitory activity of BPFRF, quercetin and standards.
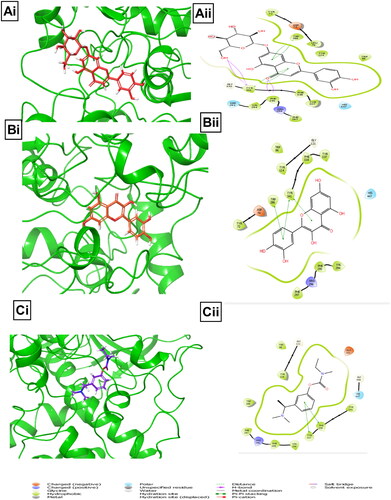
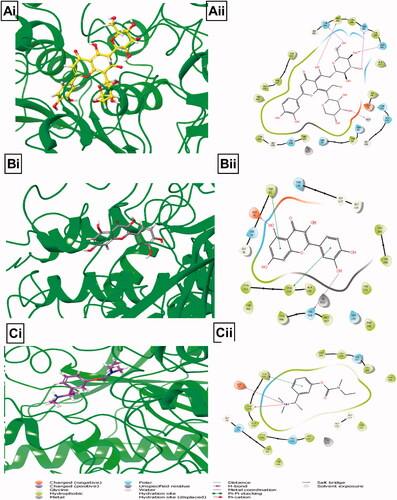
Table 3. Binding energies of BPFRF ligands and known inhibitors against AChE and BuChE drug targets.
Supplementary_Data_PHB.doc
Download ()Data availability statement
The data that support the findings of this study are available from the authors upon request.