Figures & data
Table 1. Semiquantitative PCR primer sequences (5′ to 3′).
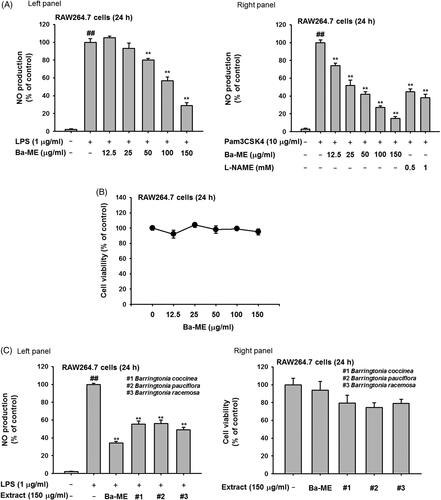
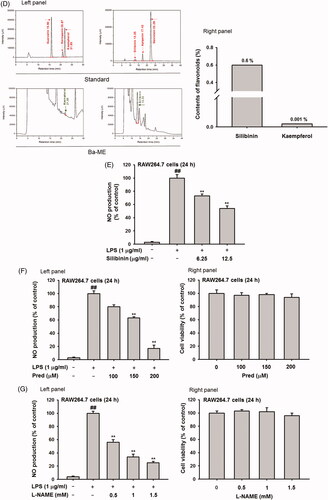
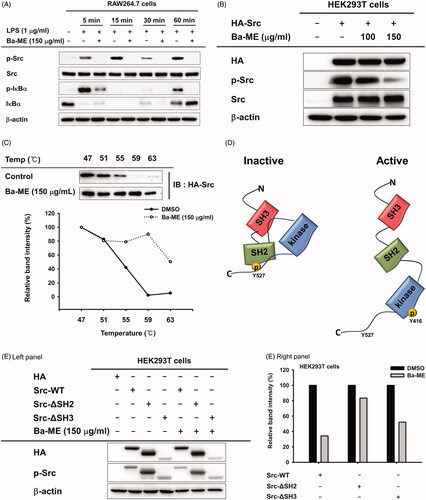
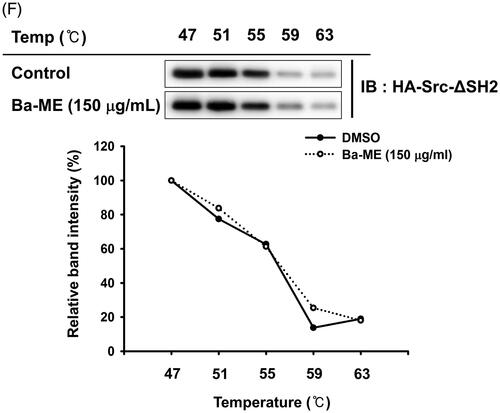
Figure 2. Expression of inflammatory genes and regulation of transcription factors after Ba-ME treatment. (A) mRNA expression levels of iNOS, COX-2, TNF-α, IL-1β, and IL-6 were determined by semiquantitative RT-PCR. (B) HEK293T cells were co-transfected with the NF-κB luciferase construct, MyD88 or TRIF plasmids, and a β-gal plasmid (as a transfection control). Cells were treated with Ba-ME (0–150 µg/mL) for 24 h. Luciferase activity was determined using a luminometer. (C) Expression levels of the phosphorylated forms of p50 and p65 in RAW264.7 cells were examined by western blotting. ##p < 0.01 compared with the normal group; *p < 0.05 and **p < 0.01 compared with the control group.
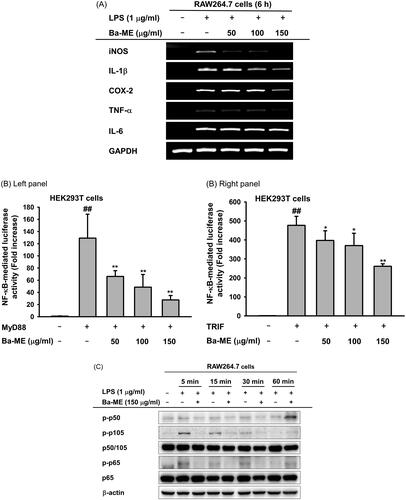
Figure 4. Anti-inflammatory effect of Ba-ME on HCl/EtOH-induced gastritis in mice. (A) Mice were orally injected with Ba-ME (50 and 100 mg/kg) or ranitidine (40 mg/kg) four times before oral administration of HCl/EtOH. At 1 h after administration of HCl/EtOH, the stomachs of the mice were excised, and gastric lesions in the stomachs were measured with ImageJ. The gastritis index of the control group (inducer alone) is represented as 100%. (B) mRNA expression levels of COX-2 and IL-1β in the stomach tissues of mice treated with HCl/EtOH were determined by semiquantitative RT-PCR. (C) Levels of the total and phosphorylated forms of Src in stomach tissues of mice treated with HCl/EtOH were examined by western blotting. ##p < 0.01 compared with the normal group and **p < 0.01 compared with the control group.
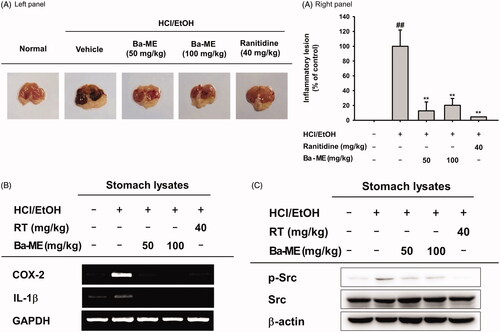
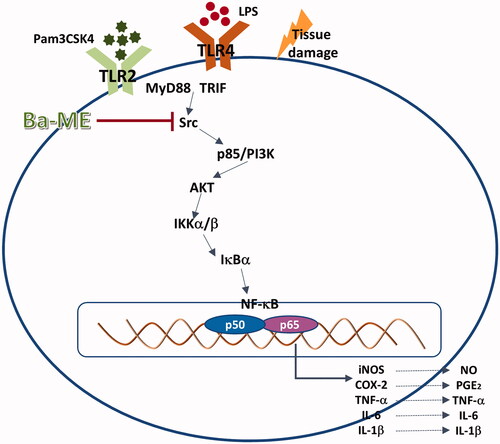
Figure 5. Molecular mechanisms underlying the anti-inflammatory action of Ba-ME. Ba-ME suppresses the phosphorylation of NF-κB pathway components and the translocation of p65 and p50 into the cell nucleus, thereby inhibiting inflammatory responses. LPS, lipopolysaccharide; TLR, toll-like receptor; MyD88, myeloid differentiation factor 88; TRIF, toll-receptor-associated activator of interferon; Ba-ME, Barringtonia angusta methanol extract; PI3K, phosphoinositide 3 kinase; IκBα, inhibitor of κBα..