Figures & data
Figure 2. Typical chromatograms of different samples (A1) Tadehaginoside in blank plasma; (A2) Tadehaginoside in blank matrix (spleen); (A3) Plasma sample at 5 min after intravenous administration of tadehaginoside; (A4) Kidney sample at 30 min after intravenous administration of tadehaginoside; (B1) Blank plasma spiked with IS; (B2) Blank matrix (spleen) spiked with IS; (B3) Plasma sample at 5 min after intravenous administration spiked with tadehaginoside and IS; (C1) HYD in Blank plasma; (C2) Plasma sample at 5 min after intravenous administration spiked with HYD; (C3) Plasma sample at 5 min after intragastric administration 5 min spiked with HYD.
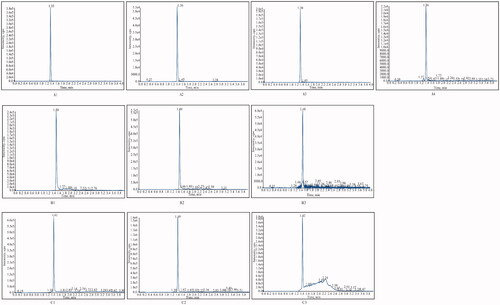
Table 1. Parameters of standard curves of tadehaginoside and HYD in the rats as determined by LC-MS/MS during method validation.
Table 2. Accuracy, precision, matrix effect and recovery of the LC-MS/MS method to determined tadehaginoside and HYD in rat plasma and various tissues (n = 6).
Table 3. Stability of the tadehaginoside and HYD in rat plasma and various tissues under different storage conditions.
Figure 3. Mean plasma concentration–time curves of tadehaginoside and p-hydroxycinnamic acid after (A), (C) intragastric administration (25 mg/kg); (B), (D) intravenous administration (5 mg/kg) to rats.
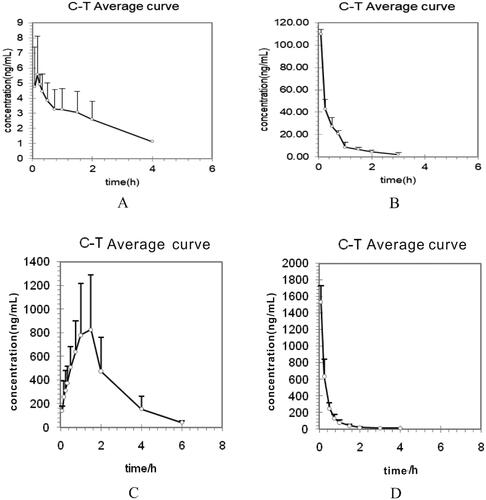
Table 4. Pharmacokinetic parameters of tadehaginoside and HYD after intragastric and intravenous administrations.

![Figure 5. The mass spectrum of tadehaginoside [(A), Q1-scan; (B) Full-scan], p-hydroxycinnamic acid [(C), Q1-scan; (D) Full-scan] and quercetin [(E), Q1-scan; (F) Full-scan].](/cms/asset/365b0ce7-6dba-4129-90d5-d25a795a1dfc/iphb_a_1990354_f0005_c.jpg)