Figures & data
Figure 1. Vitexin suppresses endometrial cancer cell viability. (A) The chemical structure of vitexin. (B) CCK-8 assay measured the cell viability of HEC-1B after treatment with vitexin (0, 1.25, 2.5, 5, 10, 20, 40, 80 μM) for 24 h. (C) CCK-8 assay measured the cell viability of Ishikawa after treatment with vitexin (0, 1.25, 2.5, 5, 10, 20, 40, 80 μM) for 24 h. (D) CCK-8 assay measured the cell viability of HESCs after treatment with vitexin (0, 1.25, 2.5, 5, 10, 20, 40, 80 μM) for 24 h. (E) CCK-8 assay measured the cell viability of HEC-1B, Ishikawa and HESCs after treatment with 10 μM vitexin for 24, 48 and 72 h. *p < 0.05; **p < 0.01; ***p < 0.001 vs. vitexin 0 μM group or 0 h group.
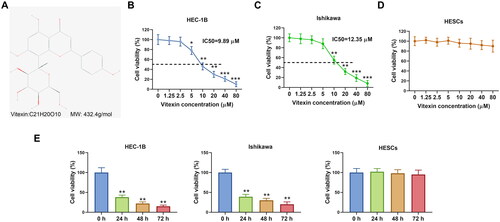
Figure 2. Vitexin suppresses endometrial cancer cell proliferation. (A) Cell proliferation of HEC-1B and Ishikawa after treatment with vitexin (0, 5, 10, 20 μM) for 24 h was assessed using EdU staining assay. (B) The protein levels of Ki-67 and PCNA after treatment with vitexin (0, 5, 10, 20 μM) for 24 h were measured using Western blots. *p < 0.05; **p < 0.01 vs. vitexin 0 μM group.
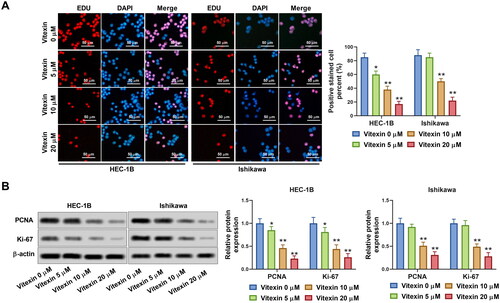
Figure 3. Vitexin suppresses endometrial cancer cell angiogenesis in vitro. HEC-1B and Ishikawa cells were treated with vitexin (0, 5, 10, 20 μM) for 24 h. (A) Angiogenesis ability of HEC-1B and Ishikawa cells was evaluated using the tube formation assay. (B) The protein levels of VEGFA and FGF2 were measured using Western blots. *p < 0.05; **p < 0.01 vs. vitexin 0 μM group.
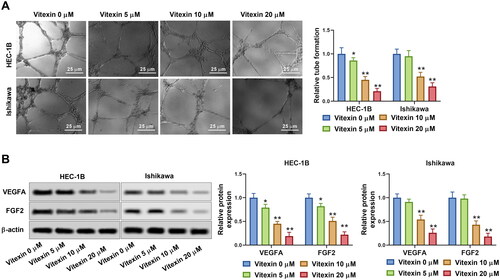
Figure 4. Vitexin suppresses endometrial cancer cell stemness capacity. HEC-1B and Ishikawa cells were treated with vitexin (0, 5, 10, 20 μM) for 24 h. (A) Stemness capacity of HEC-1B and Ishikawa cells was evaluated using sphere formation assay. (B) The protein levels of OCT4 and Nanog were measured using Western blots. *p < 0.05; **p < 0.01 vs. vitexin 0 μM group.
Figure 5. Vitexin suppresses activation of the PI3K/AKT pathway. (A) The protein levels of p-PI3K, total PI3K, p-AKT (S473), p-AKT (T308), and total AKT after treatment with vitexin (0, 5, 10, 20 μM) for 24 h were measured using Western blots. *p < 0.05; **p < 0.01 vs. vitexin 0 μM group.
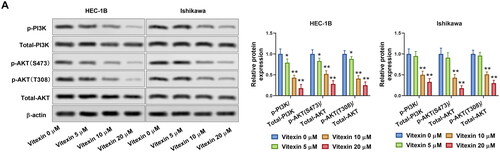
Figure 6. Vitexin suppresses the malignant phenotype of endometrial cancer via inhibiting the PI3K/AKT pathway. (A) The protein levels of p-PI3K, total PI3K, p-AKT (S473), p-AKT (T308), and total AKT after treatment with 20 μM vitexin and 20 μM 740Y-P for 24 h were measured using Western blots. (B) EdU staining assay determined the cell proliferation of HEC-1B cells after treatment with 20 μM vitexin and 20 μM 740Y-P for 24 h. (C) Tube formation assay assessed the angiogenesis ability of HEC-1B cells after treatment with 20 μM vitexin and 20 μM 740Y-P for 24 h. (D) Sphere formation assay evaluated the stemness capacity of HEC-1B after treatment with 20 μM vitexin and 20 μM 740Y-P for 24 h. **p < 0.01 vs. control group.
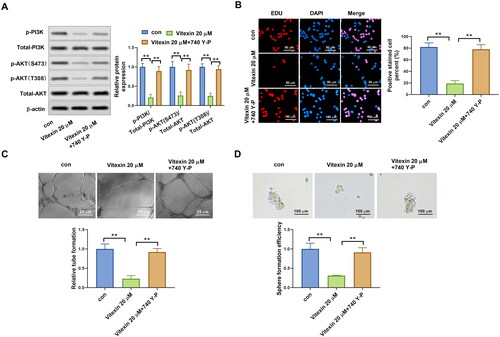
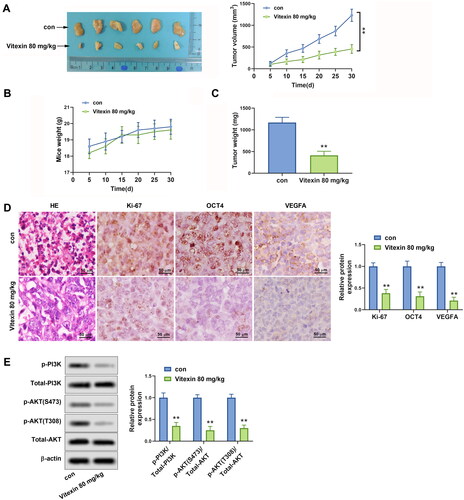
Figure 7. Vitexin suppresses tumour growth of endometrial cancer in vivo. HEC-1B cells (1 × 107) were injected subcutaneously into mice of the control group and Vitexin group. Each group contained 6 mice. The mice in the Vitexin group were injected intraperitoneally with 80 mg/kg of vitexin twice weekly for 4 weeks. The mice in the control group were given the same amount of normal saline. (A) Images of the xenograft tumours from all mice at the endpoint and the tumour volumes of mice after treatment with vitexin. (B) The body weights of mice after treatment with vitexin. (C) The tumour weights of mice after treatment with vitexin. (D) The proliferation of tumour cells was evaluated by HE staining, and the levels of Ki-67, OCT4, and VEGFA in tumour tissues after treatment with vitexin were measured using Immunohistochemistry. (E) The protein levels of p-PI3K, total PI3K, p-AKT (S473), p-AKT (T308), and total AKT in tumour tissues after treatment with vitexin were determined using Western blots. **p < 0.01 vs. control group.
Supplemental Material
Download PDF (1.9 MB)Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
