Open access
303
Views
0
CrossRef citations to date
0
Altmetric
Letter to the Editor
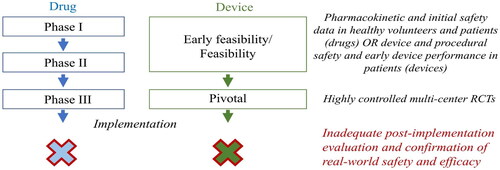
Regulatory bodies and health care systems should systematically evaluate the safety and cost-effectiveness of new cardiovascular treatments in health care registries using prospectively designed protocols
Björn Redforsa Department of Cardiology, Sahlgrenska University Hospital, Gothenburg, Sweden;b Clinical Trial Center, Cardiovascular Research Foundation, NY, USACorrespondence[email protected]
View further author information
View further author information
Article: 2343383
|
Received 04 Jan 2024, Accepted 10 Apr 2024, Published online: 02 May 2024
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.