Figures & data
Figure 1. Mean days on ECMO, Impella CP/5.0, and Temporary Central LVAD (Levitronix CentriMag) where 5.2 (±2.8), 10.2 (±6.4), and 20 (±6.2) respectively. Median TMCS duration 19.5 (14–26) days.
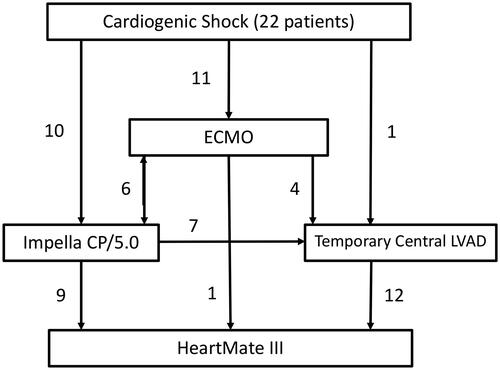
Table 1. Baseline characteristics prior to HM3 implantation.
Table 2. Laboratory parameters pre-TMCS, pre-HM3 without TMCS, and pre-HM3 with TMCS.
Table 3. Preoperative hemodynamics prior to HM3 implantation.
Table 4. Perioperative data.
Figure 2. Kaplan–Meier graph of survival censored for follow-up shows a four year survival of 80 and 82% for the TMCS and non-TMCS group.
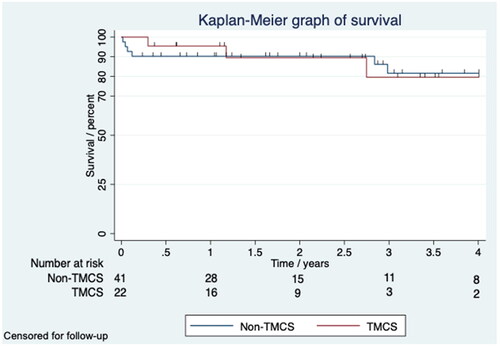
Table 5. Early postoperative outcomes and complications (<30 days).
Table 6. Adverse events after long-term follow-up (event per patient-years).
Supplemental Material
Download MS Word (14.9 KB)Data availability statement
The data are available on request to the corresponding author.
