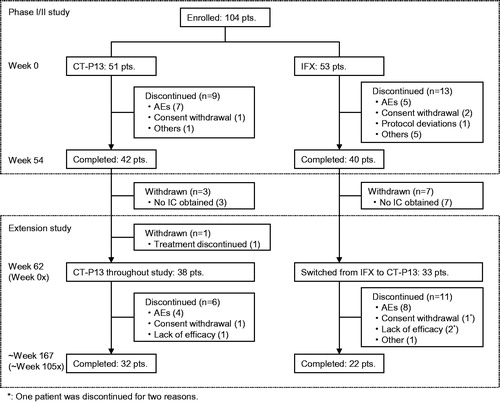
Figures & data
Table 1. Baseline patient demographics and disease characteristics—safety analysis set.
Table 2. Summary of safety—safety analysis set.
Table 3. SAEs and AEs leading to study discontinuation occurred in extension phase—safety analysis set.
Table 4. Adverse events reported in at least 5% of patients in either of the treatment groups—safety analysis set.
Figure 2. Changes in ADA status at baseline and Week 110 (48×) and the relationship between ADA status and dose of CT-P13 in patients who maintained the CT-P13 treatment throughout study (n = 38) and patients who switched from IFX to CT-P13 (n = 33) in the safety analysis set.
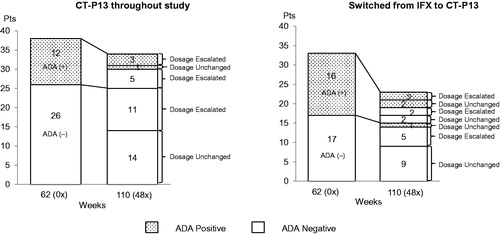
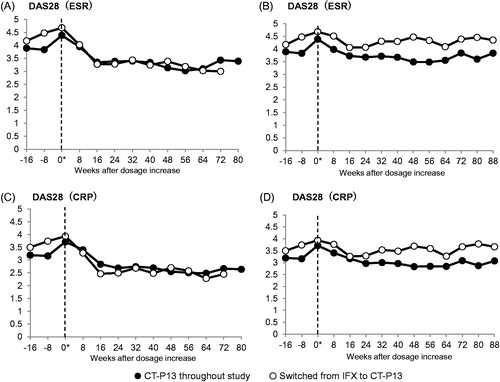
Figure 3. Changes after dose-increase in DAS28 for patients who maintained the CT-P13 treatment throughout the study (solid circle; n = 23) and patients who switched from IFX to CT-P13 (open circle, n = 16). (A) DAS28 (ESR) in the FAS without LOCF and NRI. (B) DAS28 (ESR) in the FAS with the missing data imputed by the LOCF and NRI method. (C) DAS28 (CRP) in the FAS without LOCF and NRI. (D) DAS28 (CRP) in the FAS with the missing data imputed by the LOCF and NRI method. “0*” denotes the time of dose-increase.