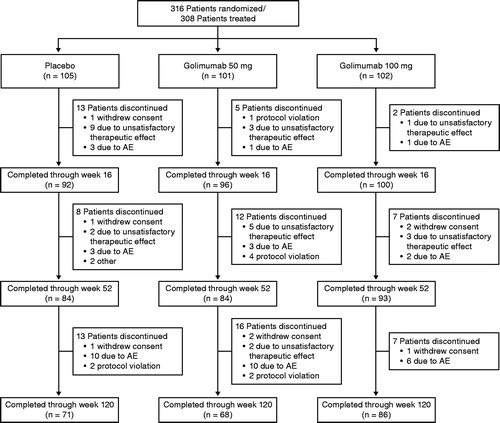
Figures & data
Figure 2. The proportions of patients with an ACR20 (a), ACR50 (b), and ACR70 (c) response through week 120. All placebo patients crossed over to golimumab at week 16 (dashed line). ACR20/50/70, ≥ 20%/50%/70% improvement in American College of Rheumatology criteria.
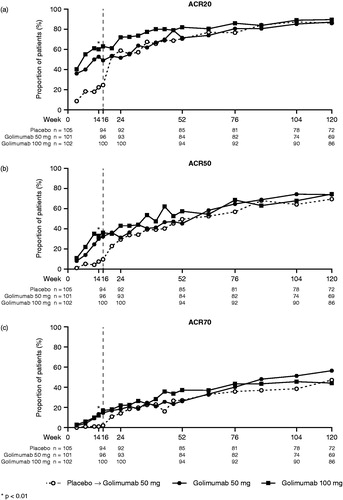
Table 1. Clinical efficacy results at weeks 52, 104, and 120.
Figure 3. The proportions of patients achieving remission by CDAI (a), SDAI (b), and Boolean (c) criteria through week 120. All placebo patients crossed over to golimumab at week 16 (dashed line). CDAI: clinical disease activity index; SDAI: simplified disease activity index.
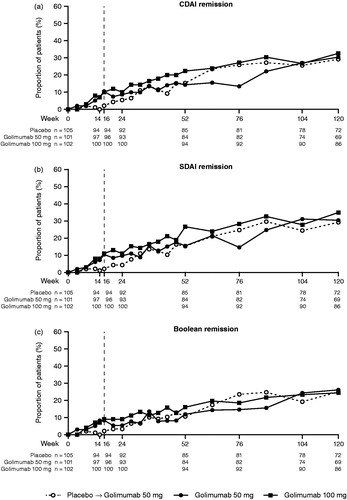
Figure 4. Change in Total vdH-S score in each treatment group at week 120 for all patients (a), patients who initiated methotrexate (b), and patients who remained on monotherapy (c). vdH-S, van der Heijde modification of the Sharp score.
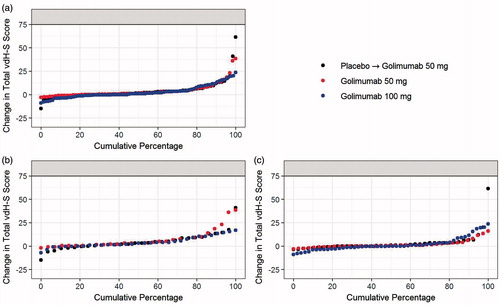
Table 2. Radiographic results through week 120.
Table 3. Proportion of patients receiving concomitant MTX and/or prednisolone through week 120.
Figure 5. Mean prednisolone dose per day through week 120 for all the patients (a), patients who initiated methotrexate (b), and patients who remained on monotherapy (c). First administration refers to week 0 administration of placebo or golimumab.
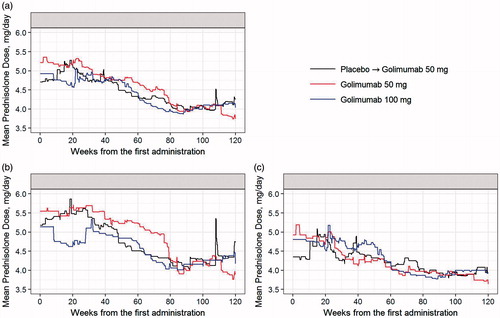
Table 4. Adverse events through week 120.