Figures & data
Figure 1. Patient disposition in the 26-week postmarketing surveillance program. CDAI: Clinical Disease Activity Index; CRF: case report form; DAS28-ESR: Disease Activity Score in 28 joints as measured by erythrocyte sedimentation rate; IV: intravenous; RA: rheumatoid arthritis; TCZ: tocilizumab. aPatient data up to time of discontinuation were available for DAS28-ESR and CDAI calculations.
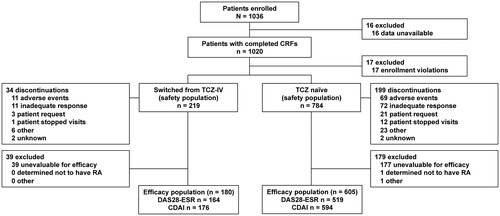
Table 1. Patient demographic and baseline characteristics.
Table 2. Adverse events and serious adverse events.
Table 3. Adverse events per 100 patient years.
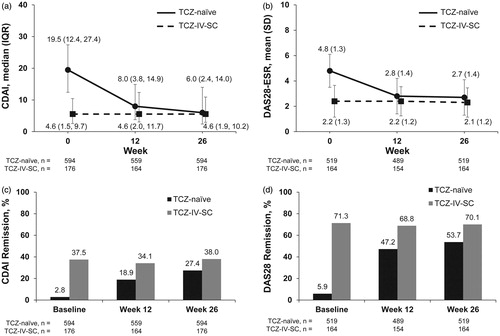
Figure 2. CDAI, DAS28-ESR, and remission by group over 26 weeks. Median (IQR) CDAI (a) and mean (SD) DAS28-ESR (b) from baseline to week 26 for patients in the TCZ-naïve and TCZ-IV-SC groups. CDAI (c) and DAS28-ESR (d) remission rates from baseline to week 26 in the TCZ-naïve and TCZ-IV-SC groups. CDAI: Clinical Disease Activity Index; DAS28-ESR: Disease Activity Score in 28 joints as measured by erythrocyte sedimentation rate; IQR: interquartile range; SD: standard deviation; TCZ: tocilizumab; TCZ-IV-SC: patients who switched from intravenous to subcutaneous tocilizumab.