Figures & data
Table 1. Baseline characteristics for overall and Japanese patient populations in studies RA-BEAM and RA-BEGIN.
Figure 1. Change from baseline in (a) Pain VAS, (b) duration of MJS, (c) HAQ-DI, (d) FACIT-F, (e) SF-36 PCS, and (f) SF-36 MCS by SDAI-defined disease activity at Week 24 for overall and Japanese populations of Study RA-BEAM. For Pain VAS, HAQ-DI, and duration of MJS, lower values/scores indicate better outcomes. For FACIT-F, SF-36 PCS, and SF-36 MCS, higher scores indicate better outcomes. HDA: SDAI >26; MDA: 11 < SDAI ≤26; LDA: 3.3 < SDAI ≤11; Rem: SDAI ≤3.3. *p<.05 versus HDA; †p<.05 versus MDA; ‡p<.05 versus LDA. FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ?DI: Health Assessment Questionnaire-Disability Index; HDA: high disease activity; LDA: low disease activity; LS: least squares; MCS: Mental Component Score; MDA: moderate disease activity; MJS: morning joint stiffness; PCS: Physical Component Score; Rem: remission; SDAI: Simplified Disease Activity Index; SF-36: Medical Outcomes Study Short Form 36 Health Survey version 2; VAS: visual analog scale.
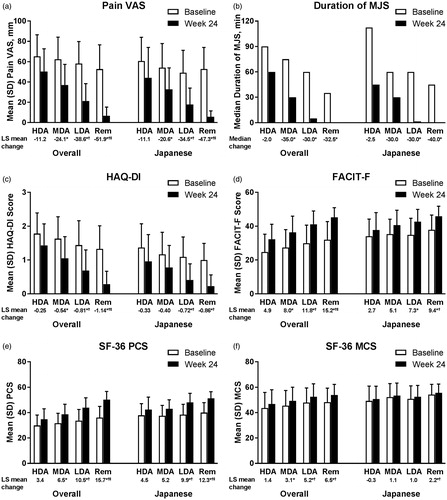
Figure 2. Change from baseline in (a) Pain VAS, (b) duration of MJS, (c) HAQ-DI, (d) FACIT-F, (e) SF-36 PCS, and (f) SF-36 MCS by SDAI-defined disease activity at Week 24 for overall and Japanese populations of Study RA-BEGIN. For Pain VAS, HAQ-DI, and duration of MJS, lower values/scores indicate better outcomes. For FACIT-F, SF-36 PCS, and SF-36 MCS, higher scores indicate better outcomes. HDA: SDAI >26; MDA: 11 < SDAI ≤26; LDA: 3.3 < SDAI ≤11; Rem: SDAI ≤3.3. *p<.05 versus HDA; †p<.05 versus MDA; ‡p<.05 versus LDA. FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ?DI: Health Assessment Questionnaire-Disability Index; HDA: high disease activity; LDA: low disease activity; LS: least squares; MCS: Mental Component Score; MDA: moderate disease activity; MJS: morning joint stiffness; PCS: Physical Component Score; Rem: remission; SDAI: Simplified Disease Activity Index; SF-36: Medical Outcomes Study Short Form 36 Health Survey version 2; VAS: visual analog scale.
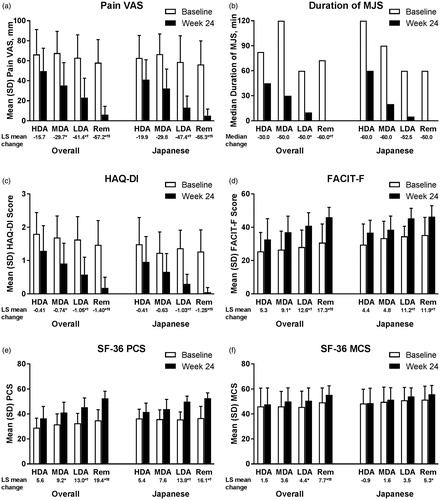
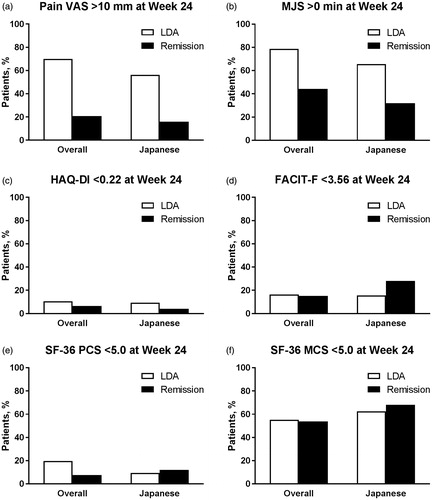
Figure 3. Proportion of patients (a) with Pain VAS >10 mm, with (b) MJS >0 min, who did not achieve the MCID for (c) HAQ-DI (change <0.22) or (d) FACIT-F (change <3.56), or who did not achieve the MCID for (e) SF-36 PCS (change <5.0) or (f) SF-36 MCS (change <5.0) at Week 24 by SDAI-defined disease activity (LDA and remission) at Week 24 for overall and Japanese populations of Study RA-BEAM. LDA: 3.3 < SDAI ≤11; Remission: SDAI ≤3.3; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ?DI: Health Assessment Questionnaire-Disability Index; LDA: low disease activity; MCS: Mental Component Score; MCID: minimum clinically important difference; MJS: Morning Joint Stiffness; PCS: Physical Component Score; SDAI: Simplified Disease Activity Index; SF-36: Medical Outcomes Study Short Form 36 Health Survey version 2; VAS: visual analog scale.
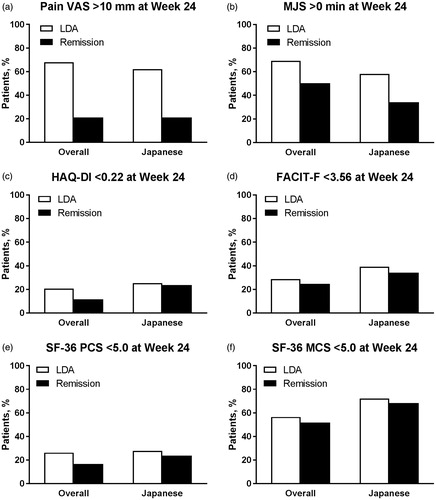
Figure 4. Proportion of patients (a) with Pain VAS >10 mm, with (b) MJS >0 min, who did not achieve the MCID for (c) HAQ-DI (change <0.22) or (d) FACIT-F (change <3.56), or who did not achieve the MCID for (e) SF-36 PCS (change <5.0) or (f) SF-36 MCS (change <5.0) at Week 24 by SDAI-defined disease activity (LDA and remission) at Week 24 for overall and Japanese populations of Study RA-BEGIN. LDA: 3.3 < SDAI ≤11; Remission: SDAI ≤3.3; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ?DI: Health Assessment Questionnaire-Disability Index; LDA: low disease activity; MCS: Mental Component Score; MCID: minimum clinically important difference; MJS: Morning Joint Stiffness; PCS: Physical Component Score; SDAI: Simplified Disease Activity Index; SF-36: Medical Outcomes Study Short Form 36 Health Survey version 2; VAS: visual analog scale.