Figures & data
Table 1. Demographic and baseline clinical characteristics.
Figure 1. Kaplan–Meier survival analysis in the safety analysis set. Retention rates of NEG and EG in the safety analysis set (n = 3,882) were analyzed by Kaplan–Meier analysis. (a) Retention rates based on discontinuations for any reason. (b) Retention rates based on discontinuations caused by adverse events, surgery, or death. (c) Retention rates based on discontinuations caused by insufficient effectiveness. NEG: non-elderly group; EG: elderly group.
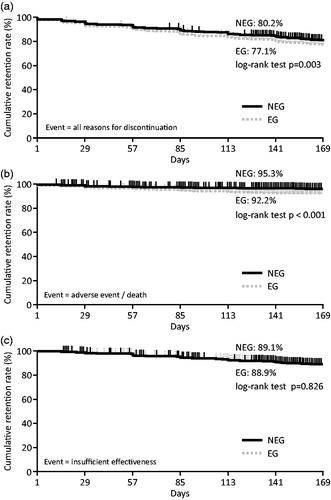
Figure 2. Changes of DAS28-CRP in the NEG and EG over 24 weeks. Changes in DAS28-CRP scores in the NEG and EG in the effectiveness analysis set II were analyzed. Disease activity was categorized into remission (DAS28-CRP ≤2.6), low disease activity (DAS28-CRP >2.6–≤3.2), moderate disease activity (DAS28-CRP >3.2–≤5.1), and high disease activity (DAS28-CRP >5.1). Changes in DAS28-CRP (left panel) and ΔDAS28-CRP (right panel) at the indicated time point are shown in b, c, and d. (a) Percent changes in the distribution of patients in each disease activity category are shown. Statistical significance of remission and low disease activity between the NEG and EG at Week 24 was determined using the Wilcoxon two-sample test. (b) Changes in total patients. (c) Changes in patients stratified by disease activity category at baseline. (d) Changes in patients who were treated with or without concomitant MTX at baseline. DAS28-CRP, Disease Activity Score 28 based on C-reactive protein; MTX: methotrexate; NEG: non-elderly group; EG: elderly group.
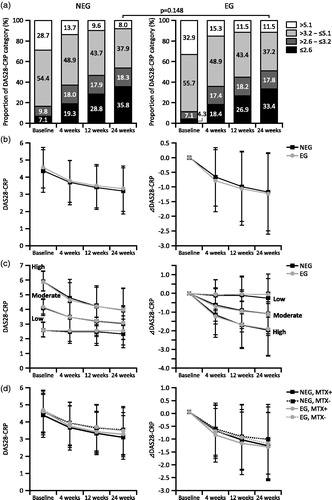
Figure 3. Patient distribution in risk-benefit model analysis on the probability matrix. The probability of infection (risk) and of achievement of DAS28-CRP improvement >1.2 (benefit) of each patient included in the risk-benefit analysis set were calculated as described in the “Methods section”. Each patient was mapped on the risk-benefit matrix and belonged to one of six groups (defined in the Results section). DAS28-CRP, Disease Activity Score 28 based on C-reactive protein; NEG: non-elderly group; EG: elderly group.
Table 2. Patient demographic and clinical characteristics for the risk-benefit model analysis (n = 2,345).
Table 3. Number of events and proportions of patients in each group in the risk-benefit model analysis (n = 2,345).
