Figures & data
Figure 1. Patient disposition. AE: adverse event; TCZ-SC: subcutaneous tocilizumab; qw: weekly; q2w: every other week. a One patient was excluded due to having no evaluable measurements after study drug administration.
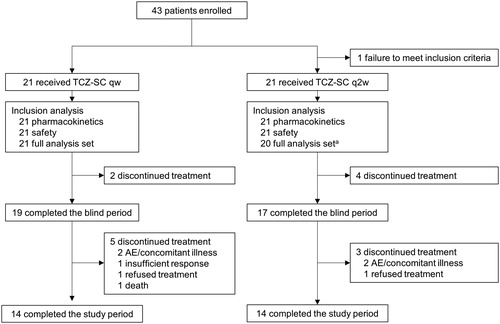
Table 1. Summary of adverse events (AEs) and serious adverse events (SAEs)Table Footnotea from baseline to week 52 by body system.
Figure 2. Mean change in DAS28-ESR and CDAI from baseline to week 52. (a) Mean change in DAS28-ESR from baseline to week 52. (b) Mean change in CDAI from baseline to week 52. CDAI: Clinical Disease Activity Index; DAS28-ESR: Disease Activity Score based on 28 joints using erythrocyte sedimentation rate; TCZ-SC: subcutaneous tocilizumab; qw: weekly; q2w: every other week. Error bars represent the standard deviation.
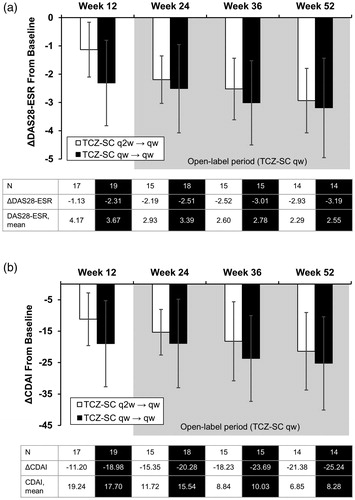
Figure 3. DAS28-ESR and CDAI response at weeks 12 and 52. (a) DAS28-ESRa response at weeks 12 and 52. (b) CDAIb response at weeks 12 and 52. CDAI: Clinical Disease Activity Index; DAS28-ESR: Disease Activity Score based on 28 joints using erythrocyte sedimentation rate; HDA: high disease activity; LDA: low disease activity; MDA: moderate disease activity; TCZ-SC: subcutaneous tocilizumab; qw: weekly; q2w: every other week. a Remission was defined as DAS28-ESR <2.6; LDA as DAS28-ESR ≥2.6 to ≤3.2; MDA as DAS28-ESR >3.2 to ≤5.1; and HDA as DAS28-ESR >5.1. bRemission was defined as CDAI ≤2.8; LDA as CDAI >2.8 to ≤10; MDA as CDAI >10 to ≤22; and HDA as CDAI >22.
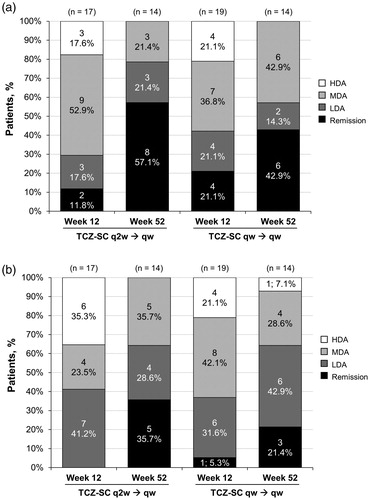
Table 2. Mean change in DAS28-ESR from baseline to week 12 by subgroup.
Supplemental Material
Download MS Word (28.6 KB)Data sharing statements
We provide qualified researchers access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details of Chugai’s Data Sharing Policy are available here (www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html).
