Figures & data
Figure 1. Patient disposition. Three patients were excluded as they did not meet the inclusion criteria (not meeting the CASPAR criteria, n = 1; not having received treatment at a stable dose of NSAIDs, DMARDs, or oral corticosteroids for at least 4 weeks before study start, n = 2). CASPAR: ClASsification criteria for Psoriatic Arthritis; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: nonsteroidal anti-inflammatory drugs.
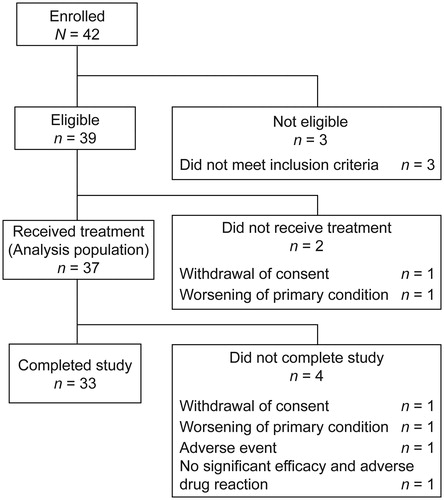
Table 1. Patient demographics and baseline characteristics (FAS population).
Figure 2. Efficacy outcomes during treatment of psoriatic arthritis with adalimumab (FAS population). (a) Percentage of patients with psoriatic arthritis who met the ACR20, ACR50, and ACR70 response criteria at weeks 12 and 24. (b) Subanalysis of the percentage of patients with psoriatic arthritis who met the ACR20, ACR50, and ACR70 response criteria at weeks 12 and 24 by previous use of biologics. (c) Percentage of patients with psoriatic arthritis who met the PASI50, PASI75, and PASI90 response criteria at weeks 12 and 24. (d) Subanalysis of the percentage of patients with psoriatic arthritis who met the PASI50, PASI75, and PASI90 response criteria at weeks 12 and 24 by previous use of biologics. (e) PGA scores in patients with psoriatic arthritis at weeks 12 and 24. ACR: American College of Rheumatology; CI: confidence interval; FAS: full analysis set; PASI: Psoriasis Area and Severity Index; PGA: Physician’s Global Assessment.
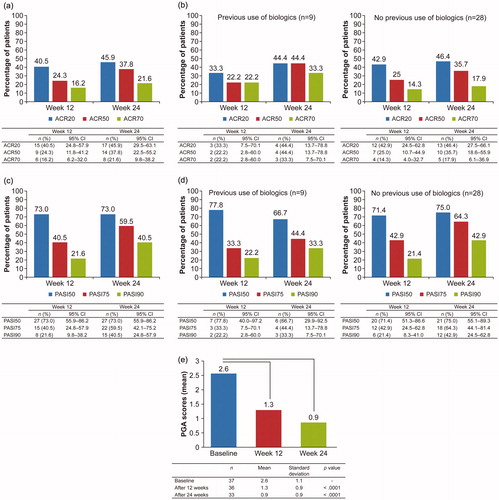
Figure 3. DAS-28CRP (a) and BASDAI (b) scores at weeks 12 and 24 (FAS population). BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; DAS-28CRP: Disease Activity Score-28 CRP; FAS: full analysis set.
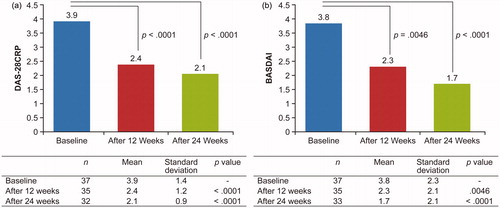
Table 2. Serum biomarker levels at weeks 12 and 24.
Figure 4. (a) HAQ-DI at weeks 12 and 24. (b) SF-36 scores at weeks 12 and 24. (c) DLQI at weeks 12 and 24 (FAS population). DLQI: Dermatology Life Quality Index; FAS: full analysis set; HAQ-DI: Health Assessment Questionnaire Disability Index; SF-36: Short Form Survey 36 items.
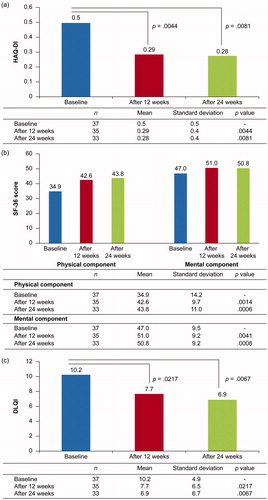
Table 3. Commonly (≥5%) reported adverse events.
