Figures & data
Figure 1. Enrollment, disposition, and pathologic classification in patients with multicentric Castleman disease. Twenty-four patients previously received tocilizumab in the clinical trial or compassionate use for tocilizumab. Nine patients with misdiagnosed MCD (5 with malignant lymphoma, 2 with another malignancy, 1 with UCD, and 1 with autoimmune disease). *One patient was excluded for two reasons: one of the five patients with malignant lymphoma had also previously received tocilizumab.
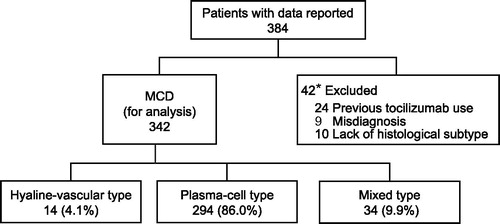
Figure 2. Age at disease onset and duration of disease in patients with multicentric Castleman disease. The distributions of age at disease onset (A) and of disease duration (B) in male (A: n = 170, B: n = 172) and female (A: n = 114, B: n = 116) patients with MCD in available data. Dark gray bars indicate male patients and light gray bars indicate female patients.
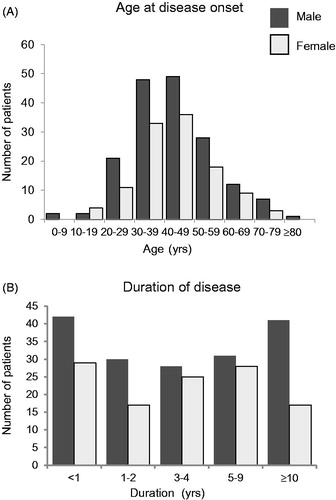
Figure 3. Mean body weight, height, and body mass index of patients with multicentric Castleman disease compared with the general Japanese population. Differences in the mean body height (A, B), weight (C, D) and body mass index (E, F) between this study population and the general Japanese population [Citation46]. The bar indicates standard deviation. Statistical analysis has not been performed because of a large difference in the sample size between this study population and the general Japanese population. Dark gray bars indicate the study population and light gray bars indicate the general Japanese population.
![Figure 3. Mean body weight, height, and body mass index of patients with multicentric Castleman disease compared with the general Japanese population. Differences in the mean body height (A, B), weight (C, D) and body mass index (E, F) between this study population and the general Japanese population [Citation46]. The bar indicates standard deviation. Statistical analysis has not been performed because of a large difference in the sample size between this study population and the general Japanese population. Dark gray bars indicate the study population and light gray bars indicate the general Japanese population.](/cms/asset/cdfaf1b0-dd2d-4a06-8fbb-c98bd9edad64/imor_a_1704983_f0003_b.jpg)
Table 1. Characteristics of the patients.
Table 2. Clinical features (≥2.0%).
Figure 4. Results of laboratory tests in patients with multicentric Castleman disease: correlation with IL-6. Laboratory tests included albumin (Alb; A), hemoglobin (Hb; B), triglycerides (TG; C), total cholesterol (T-cho; D), C-reactive protein (CRP; E), fibrinogen (Fib; F), immunoglobulin G (IgG; G), immunoglobulin A (IgA; H); immunoglobulin M (IgM; I), platelets (PLT; J), estimated glomerular filtration rate-normalized body surface area (eGFR-NOR; K). r indicates the Spearman’s product-moment correlation coefficient.
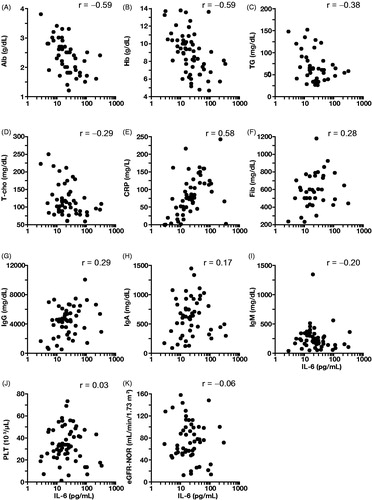
Table 3. Laboratory tests associated with multicentric Castleman disease.
Table 4. Laboratory tests: Spearman’s correlation coefficient with IL-6.
