Figures & data
Figure 1. Study design. max: maximum; q4w: every 4 weeks; sJIA: systemic juvenile idiopathic arthritis.
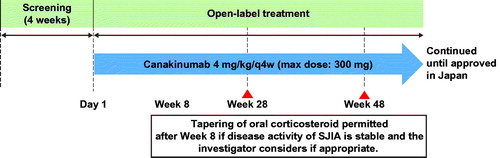
Table 1. Baseline demographic and disease characteristics.
Figure 2. ACR pediatric responses and inactive disease (with duration of morning stiffness) status. For the analyses of ACR pedi 30/50/70/90/100 criteria at week 8, missing response was imputed with non-responder regardless of the reason for missing data (NRI). No imputation was applied after week 8. †m = 19; ‡m = 16. Inactive disease was defined as no joints with active arthritis; no fever (body temperature ≤38 °C); no rheumatoid rash, serositis, splenomegaly, hepatomegaly, or generalized lymphadenopathy attributable to JIA; normal CRP; PGA of disease activity indicating no disease activity ≤10 mm; duration of morning stiffness ≤15 minutes. ACR pedi: adapted American college of rheumatology-pediatric; CRP: C-reactive protein; JIA: juvenile idiopathic arthritis; m: the total number of evaluable patients; n: number of patients with response; NRI: non-responder imputation; PGA: physician’s global assessment.
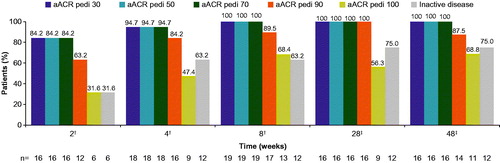
Table 2. Corticosteroid tapering at week 28, by prior use of tocilizumab.
Figure 3. Corticosteroid tapering over time from week 8. Analyses based on observed data. †Corticosteroid dose reduced from >0.8 mg/kg/day to ≤0.5 mg/kg/day, or from ≥0.5 mg/kg/day and ≤0.8 mg/kg/day by ≥0.3 mg/kg/day, or from any initial dose to ≤0.2 mg/kg/day, or any reduction from an initial dose of ≤0.2 mg/kg/day, while maintaining ACR pedi 30 criterion. ACR pedi: adapted American college of rheumatology pediatric; m: the total number of evaluable patients.
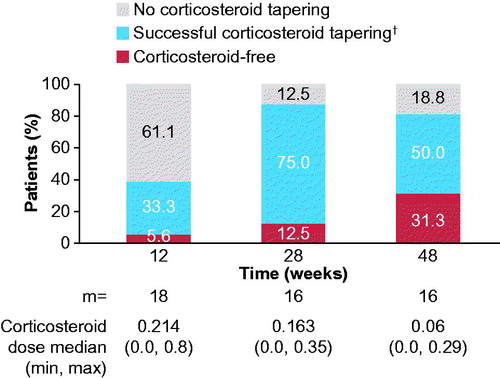
Figure 4. Change over time in the components of the ACR pediatric criteria. Analyses based on observed data. *The range of possible values for number of joints with active arthritis was 0–73. †The range of possible values for number of joints with limited range of motion was 0–69. ‡The PGA of disease activity was based on a 100-mm VAS, with higher scores indicating more active disease. §The parent’s global assessment of the patient’s overall well-being was based on a 100-mm VAS, with higher scores indicating more active disease. ![]() The level of CRP was standardized to a normal range of 0–10 mg/L. ǁPhysical function was assessed by means of the cross-culturally adapted and validated version of the CHAQ-DI, with scores ranging from 0 to 3 and higher scores indicating greater disability. ACR pedi: adapted American college of rheumatology pediatric; CHAQ-DI: childhood health assessment questionnaire – disability index; CRP: C-reactive protein; PGA: physician’s global assessment; PPGA: patients’/parents’ global assessment; sJIA: systemic juvenile idiopathic arthritis; VAS: visual analog scale.
The level of CRP was standardized to a normal range of 0–10 mg/L. ǁPhysical function was assessed by means of the cross-culturally adapted and validated version of the CHAQ-DI, with scores ranging from 0 to 3 and higher scores indicating greater disability. ACR pedi: adapted American college of rheumatology pediatric; CHAQ-DI: childhood health assessment questionnaire – disability index; CRP: C-reactive protein; PGA: physician’s global assessment; PPGA: patients’/parents’ global assessment; sJIA: systemic juvenile idiopathic arthritis; VAS: visual analog scale.
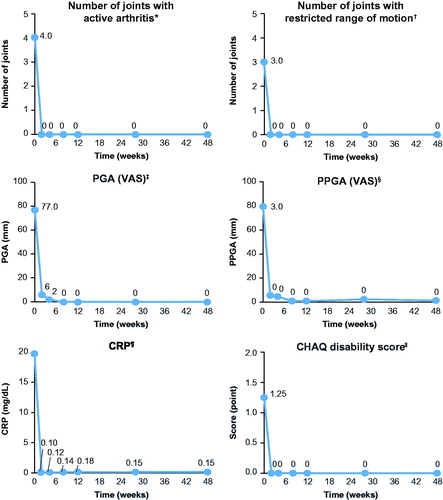
Table 3. AEs by primary system organ class (≥4 events) and preferred term (≥2 events).
