Figures & data
Figure 1. JAK family and their related cytokines. The binding of cytokines, growth factors, interferons, and peptide hormones to their respective receptors phosphorylates JAKs through the reciprocal interaction of two adjoining JAKs.
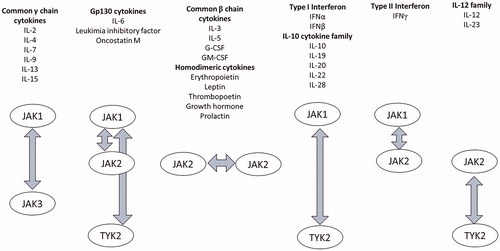
Table 1. JAK Inhibitors approved for RA (not direct comparison).
Figure 2. ACR50 achievement of placebo and each peficitinib dose in the five randomized, placebo-controlled trials. RA21 and RA22 were global phase 2b trials. RAJ1 and RAJ were conducted in Japan, and RAJ3 was conducted in Japan, Korea, and Taiwan. ACR, American College of Rheumatology. The approved peficitinib dose for rheumatoid arthritis in Japan was 100 mg and 150 mg daily.
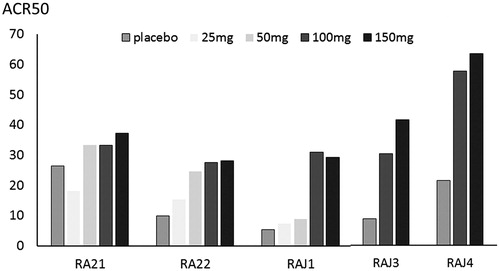
Table 2. Summary of adverse events in the five randomized, placebo-controlled trials.
