Figures & data
Box 1. Drug summary.
Table 1. Clinical trials of canagliflozin (CAN) conducted in Japan and discussed in the current review.
Figure 1. (a) Plasma concentration–time profiles after canagliflozin treatment of Japanese patients on days 1 and 16. Data are presented as mean ± standard deviation. Reproduced from [Citation40] with permission of Springer Nature. (b) Relationship between dose and renal threshold for glucose excretion (RTG0–24 h) in Japanese and non-Japanese patients with type 2 diabetes mellitus after multiple-dose treatment with canagliflozin [data taken from 40,45,46].
TA-7284-02: Japanese; DIA1023, NAP1002: non-Japanese.
![Figure 1. (a) Plasma concentration–time profiles after canagliflozin treatment of Japanese patients on days 1 and 16. Data are presented as mean ± standard deviation. Reproduced from [Citation40] with permission of Springer Nature. (b) Relationship between dose and renal threshold for glucose excretion (RTG0–24 h) in Japanese and non-Japanese patients with type 2 diabetes mellitus after multiple-dose treatment with canagliflozin [data taken from 40,45,46].TA-7284-02: Japanese; DIA1023, NAP1002: non-Japanese.](/cms/asset/ec6303b2-2b66-46c4-bc77-bb69d2c0c696/ieop_a_1473378_f0001_oc.jpg)
Table 2. Pharmacokinetic profile of canagliflozin 100 mg in patients (Japanese and non-Japanese) with T2DM [Citation40,Citation41,Citation43–Citation45].
Figure 2. Effect of canagliflozin monotherapy and combination therapy on (a) mean change in HbA1c from baseline and (b) mean percent change in body weight from baseline in placebo-controlled studies (TA-7284–04, −05, and −11) and open-label studies (TA-7284–06, −12, and −13) in Japanese patients with type 2 diabetes.
Data shown are mean ± standard deviation (TA-7284–04, −05, and −11); or mean ± 95% confidence interval (TA-7284–06, −12, and −13). Bars at the end of treatment represent last observation carried forward. HbA1c, hemoglobin A1c; CAN, canagliflozin; PBO, placebo; SU, sulfonylurea; α-GI, α-glucosidase inhibitor; TZD, thiazolidinedione; DPP-4, dipeptidyl peptidase-4 inhibitor.
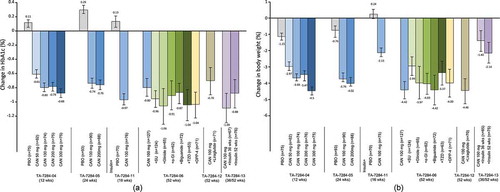
Figure 3. Effect of canagliflozin monotherapy and combination therapy on (a) mean change in systolic blood pressure from baseline, and (b) diastolic blood pressure from baseline, in placebo-controlled studies (TA-7284–04, −05, and −11) and open-label studies (TA-7284–06, −12, and −13) in Japanese patients with type 2 diabetes.
Data shown are mean ± standard deviation (TA-7284–04, −05, and −11); or mean ± 95% confidence interval (TA-7284–06, −12, and −13). Bars at the end of treatment represent last observation carried forward. SBP, systolic blood pressure; DBP, diastolic blood pressure; CAN, canagliflozin; PBO, placebo; SU, sulfonylurea; α-GI, α-glucosidase inhibitor; TZD, thiazolidinedione; DPP-4, dipeptidyl peptidase-4 inhibitor.
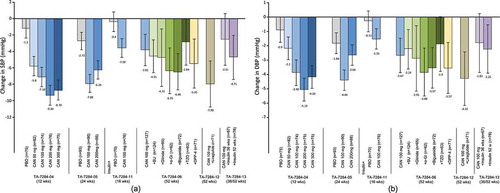
Table 3. Efficacy summary of pivotal study data about the effects of canagliflozin 100 mg on cardiometabolic parameters.
Table 4. Summary of safety data (number, % of events) for canagliflozin 100 mg.
Figure 4. Effect of canagliflozin on (a) HbA1c and (b) body weight as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes. Grey bars represent placebo, blue bars are CAN 100 mg.
Data shown are mean ± standard deviation (MT-2412-J03); or mean ± 95% confidence interval (MT-2412-J01). Bars at the end of treatment represent last observation carried forward. HbA1c, hemoglobin A1c; CAN, canagliflozin; PBO, placebo.
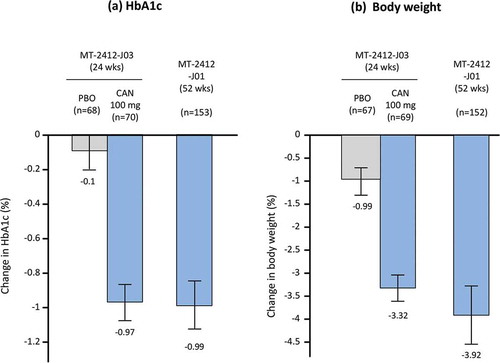
Table 5. Effect of canagliflozin in overseas studies in non-Japanese patients (for comparison).
Figure 5. Results from the CANagliflozin CardioVascular ASsessment (CANVAS) program. Reprinted from [Citation30] with permission of the Massachusetts Medical Society.
![Figure 5. Results from the CANagliflozin CardioVascular ASsessment (CANVAS) program. Reprinted from [Citation30] with permission of the Massachusetts Medical Society.](/cms/asset/b5420da3-aca9-46d0-8ec1-da7326941ee0/ieop_a_1473378_f0005_b.gif)
