Figures & data
Figure 1. Standard pairwise Bucher method used to estimate the relative efficacy of patisiran vs. tafamidis from baseline to 18 months.
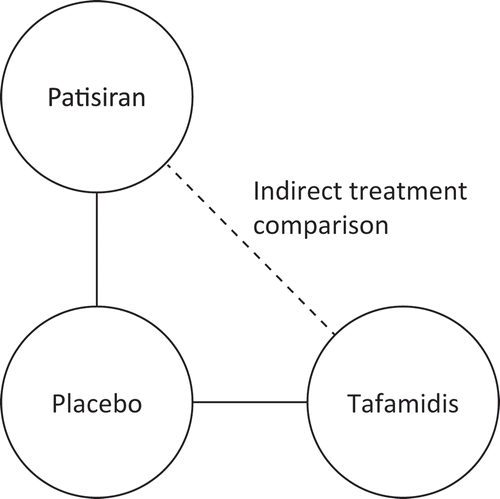
Table 1. Overview of trials included in the analysis.
Table 2. Baseline demographics and disease characteristics of patients in the APOLLO and Fx-005 trials.
Figure 2. PRISMA diagram of literature search and inclusion of randomized controlled trials (RCTs) of tafamidis.
SLR: systematic literature review.
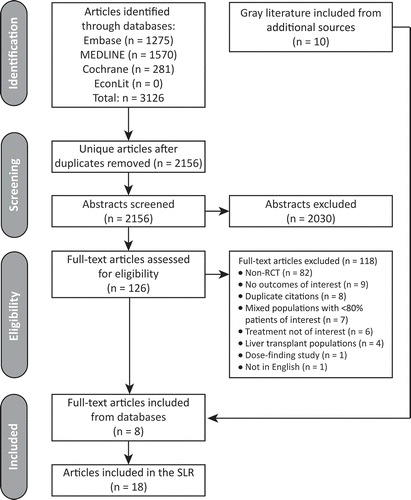
Table 3. Baseline measures of neuropathy, QoL, and nutritional status for the Fx-005 ITT population and APOLLO base-case and sensitivity analyses populations.
Figure 3. Results of the indirect treatment comparison of Fx-005 ITT population and APOLLO base-case and sensitivity analyses populations for (a) change in mean NIS-LL score; (b) change in mean NIS-LL response; (c) change in mean Norfolk QoL-DN; and (d) change in mean mBMI score. CI: confidence interval; FAP: familial amyloidotic polyneuropathy; mBMI: modified body mass index; mITT: modified intent-to-treat; NIS-LL: Neuropathy Impairment Score-Lower Limb; Norfolk QoL-DN: Norfolk Quality of Life-Diabetic Neuropathy questionnaire.
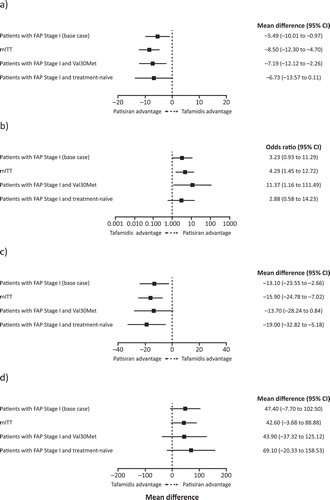
Table 4. Key safety outcomes in the APOLLO and Fx-005 trials.
