Figures & data
Figure 1. Patient disposition
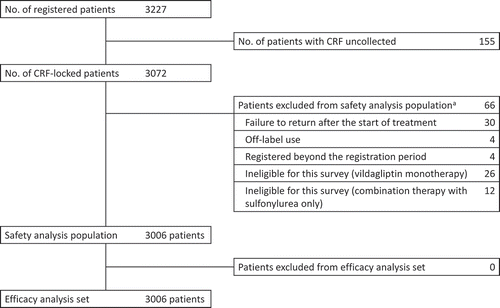
Table 1. Patient baseline characteristics
Table 2. Summary of adverse events observed in ≥5 patients
Table 3. Summary of risk management plan-related ADRs
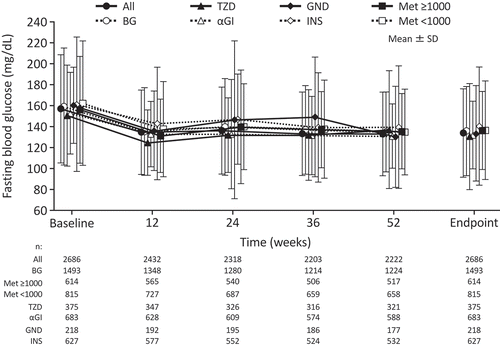
Figure 2. Changes in HbA1c over time from baseline to endpoint
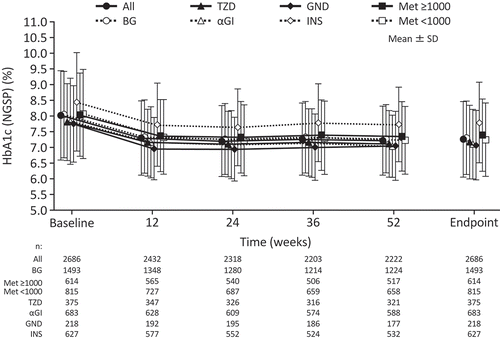
Table 4. Change in HbA1c from baseline stratified by concomitant antidiabetic medication
Table 5. Mean change in HbA1c over time stratified by patient characteristics