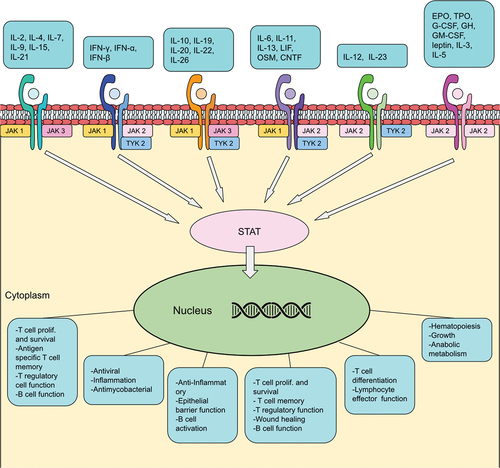
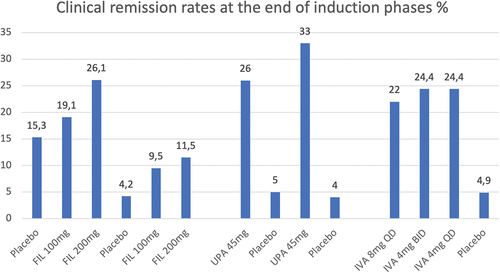
Figures & data
Table 1. Safety analysis data from Filgotinib studies. EAIR/100 PYE – Exposure-adjusted incidence rate per 100 patient-years of exposure, applies to DARWIN 3 only. AE, adverse events; SAE, serious adverse events; SI, serious infections; HZV, herpes zoster virus; VT, venous thrombosis; PE, pulmonary embolism; NMSC, non-melanoma skin cancer; Total, Total patients.
Table 2. Safety analysis data from upadacitinib studies. AE, adverse events; SAE, serious adverse events; SI, serious infections; HZV, herpes zoster virus; VT, venous thrombosis; PE, pulmonary embolism; NMSC, non-melanoma skin cancer; Total, Total patients.
Table 3. Safety analysis data from Ivarmacitinib studies. AE, adverse events; SAE, serious adverse events; SI, serious infections; HZV, herpes zoster virus; VT, venous thrombosis; PE, pulmonary embolism; Total, Total patients.