Figures & data
Table 1. Selected properties of phenanthrene and anthracene.
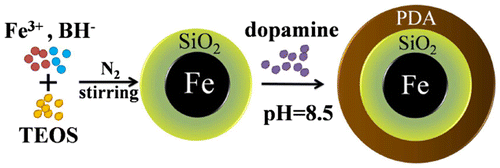
Figure 2. TEM images of (a) Fe@SiO2, (b) Fe@SiO2@PDA; size distribution histograms of (c) Fe@SiO2 and (d) Fe@SiO2@PDA; and corresponding EDS spectra of (e) Fe@SiO2 and (f) Fe@SiO2@PDA.
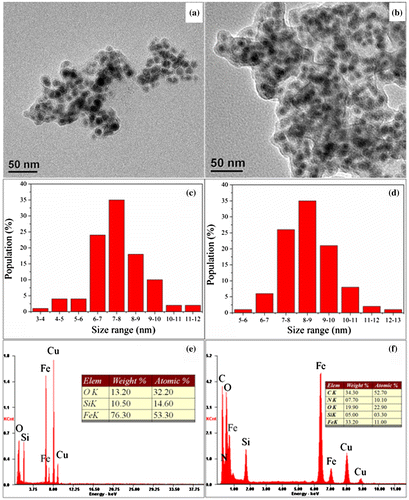
Figure 3. XRD pattern of Fe@SiO2 (a); overview (b) and high-resolution XPS spectra for Fe (c) and C (d). FTIR (e) and VSM hysteresis curves (f) of Fe@SiO2 and Fe@SiO2@PDA nanocomposites.
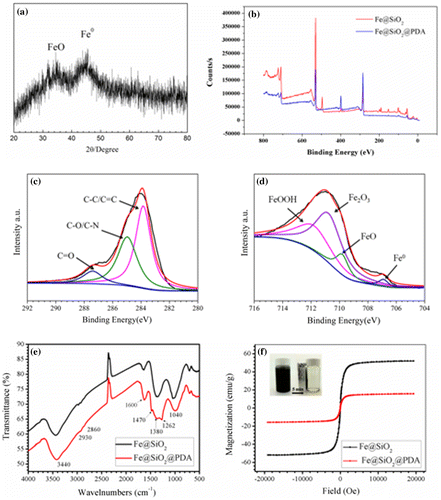
Table 2. Comparison of several iron-based nanoparticles.
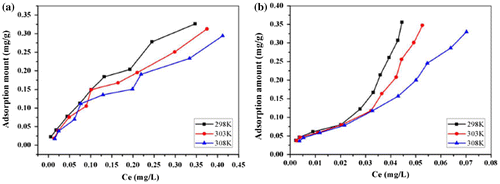
Figure 4. Influence of pH (a), ionic strength (b), HA concentration (c) and initial concentration (d) on the removal of PHE and ANT by the as-prepared Fe@SiO2@PDA.
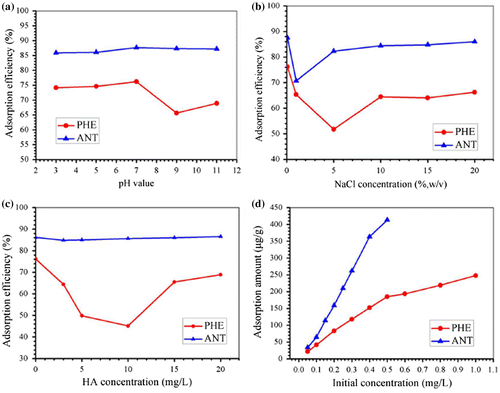
Figure 5. Kinetics of PHE and ANT removal by Fe@SiO2@PDA nanocomposites (a) and the fitted curves of pseudo-first-order model (b), pseudo-second-order model (c) and intraparticle diffusion model (d).
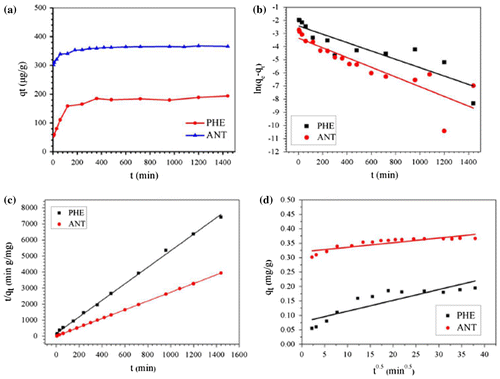
Table 3. Adsorption rate constants for kinetic models at 298 K pH 7.0.
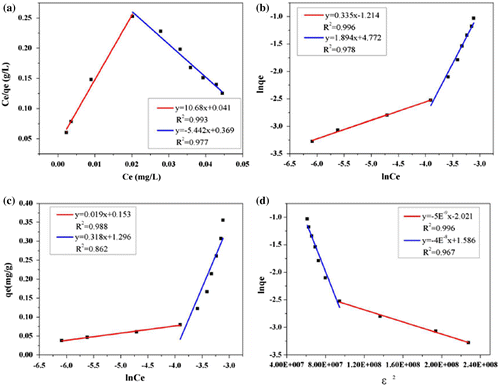
Table 4. Regression parameters of isotherm models.
Figure 7. Adsorption isotherms of ANT fitting by two stages at 298 K, (a) Langmuir, (b) Freundlich, (c) Temkin, (d) D–R model.