Figures & data
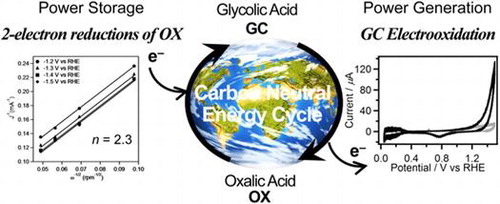
Figure 1. CVs (two-cycle scan) of V (a), Fe (b), Co (c), Cu (d), Zn (e), Mo (f), Re (g), and W (h) recorded in 0.1 M GC+0.5 M Na2SO4 (solid line) and 0.5 M Na2SO4 (broken line). The current range of graphs (a–g) is fixed to –50 to 700 mA.
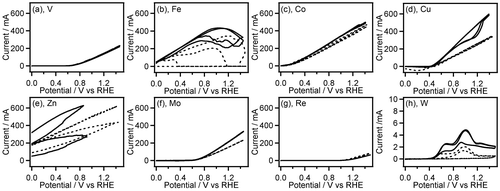
Figure 2. CVs (three-cycle scan) of Ti (a,b), Zr (c,d), Nb (e,f), Ta (g,h), and Ni (i,j) recorded in 0.1 M GC+0.5 M Na2SO4 (solid line) and 0.5 M Na2SO4 (broken line). The first (a,c,e,g,i) and the second, third (b,d,f,h,j) scan cycles of CVs are depicted separately. The current range of graphs (a–h) is fixed to –20 to 140 μA.
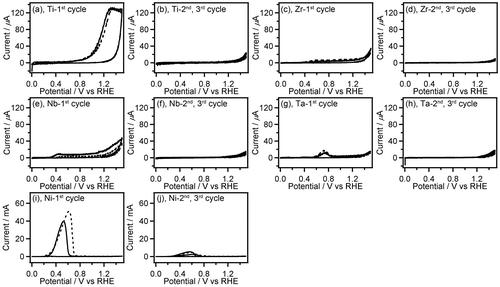
Figure 3. CVs (two-cycle scan) of Ag (a), Pd (b), Pt (c), Au (d), Rh (e,f), and Ir (g,h) recorded in 0.1 M GC+0.5 M Na2SO4 (solid line) and 0.5 M Na2SO4 (broken line). The current range of graphs (b–e,g) is fixed to –80 to 700 mA.
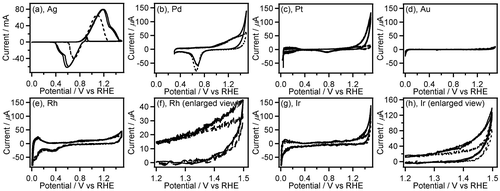
Figure 4. The colors of electrolyte solution after the CV measurements of Fe (a), Co (b), and Cu (c) electrodes in 0.1 M GC+0.5 M Na2SO4 (i) and 0.5 M Na2SO4 (ii).

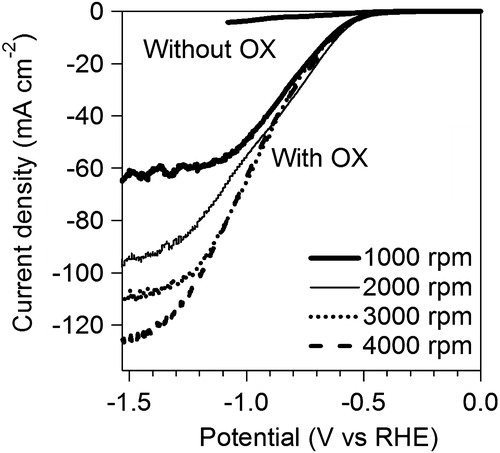
Figure 5. CVs of Pt/C in 0.5 M GC+0.5 M Na2SO4 (a) and 0.5 M GC+20 wt% KOH (b) at 50 °C. And CA curves of Pt/C in 0.5 M GC+0.5 M Na2SO4 (c) and 0.5 M GC+20 wt% KOH (d) at 50 °C.
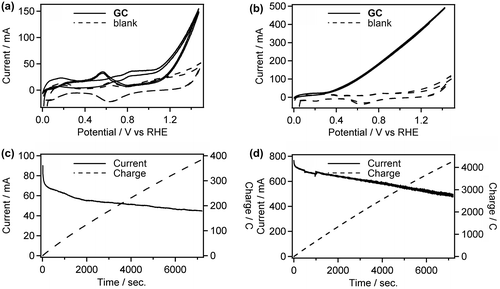
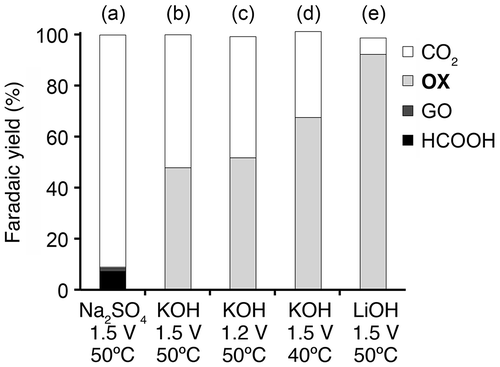
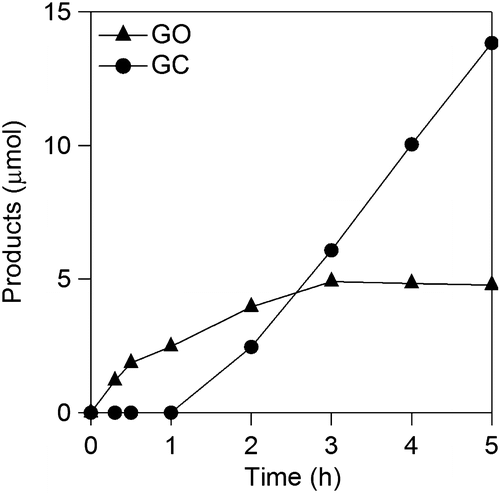
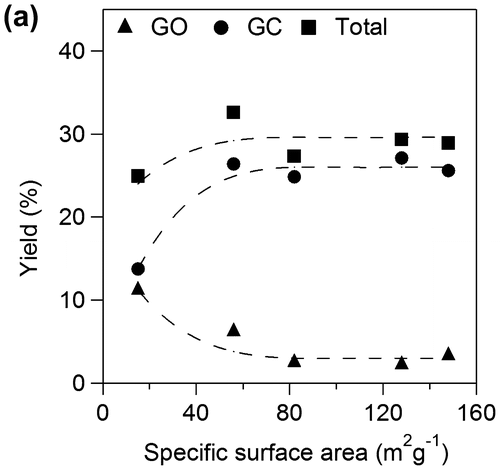
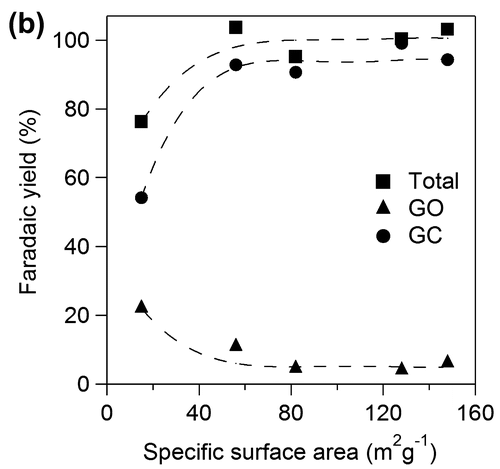
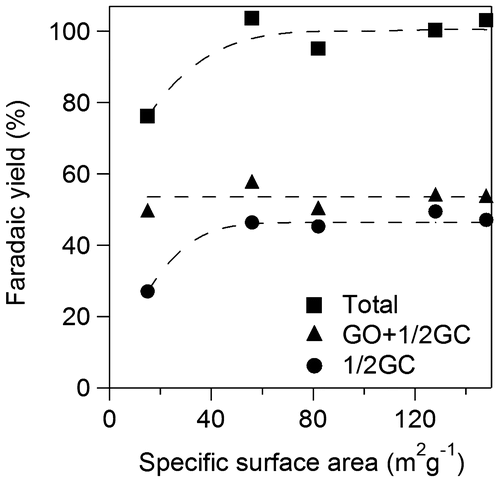
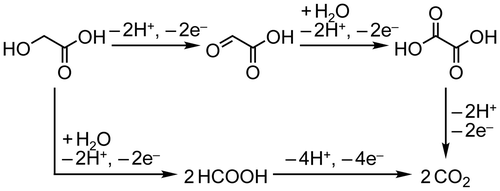
Figure 6. Faradaic yields for CO2, OX, GO, and HCOOH in GC electro-oxidation (a) at 1.5 V in 0.5 M GC and 0.5 M Na2SO4 at 50 °C, (b) at 1.5 V in 0.5 M GC and 20 wt%, i.e. 3.56 M KOH at 50 °C, (c) at 1.2 V in 0.5 M GC and 20 wt% KOH at 50 °C, (d) at 1.5 V in 0.5 M GC and 20 wt% KOH at 40 °C and (e) at 1.5 V in 0.5 M GC and 3.56 M LiOH at 50 °C.