Figures & data
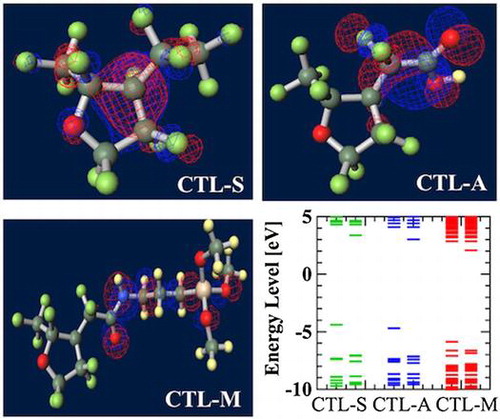
Table 1. Surface charge density of negatively charged, 15 μm-thick amorphous fluropolymers [Citation11,12].
Figure 4. (a) Principle of the inverse photoelectron spectroscopy (LEIPS), (b) Schematic of the LEIPS measurement setup.
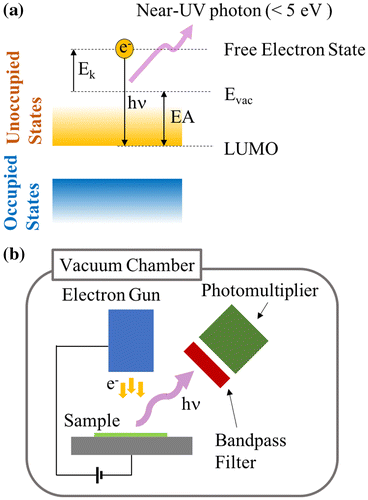
Figure 5. LEIPS results: (a) Intensity of emitted light from CTL-S with 0.15 μA of sample current, (b) Intensity of emitted light from CTL-S with 0.70 μA of sample current, (c) Thickness dependence of LEIPS onset energy for CTL-S, (d) Intensity of emitted light from CTL-A with 0.15 μA of sample current, (e) Intensity of emitted light from CTL-A with 0.70 μA of sample current, (f) Thickness dependence of LEIPS onset energy for CTL-A.
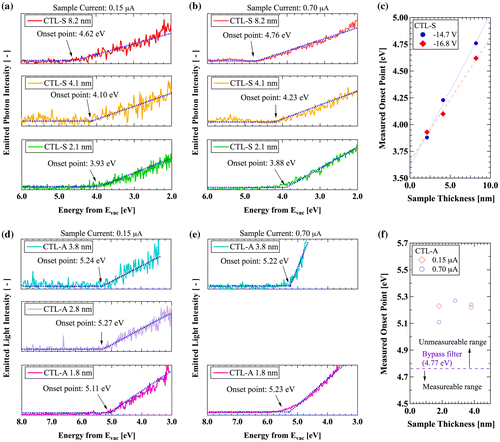
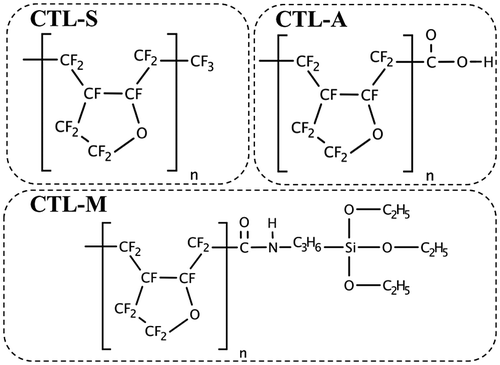
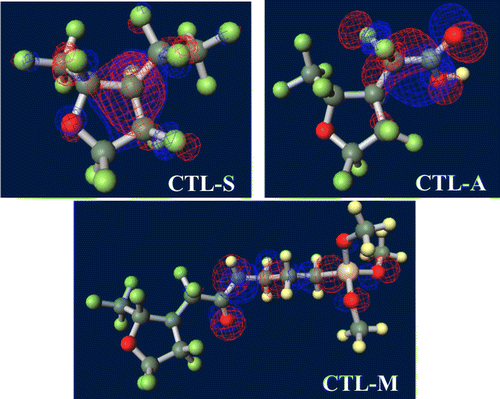
Figure 7. Ground-state structures of (a) PE, (b) PTFE, (c) PTFE, (d) CTL-S, (e) CTL-A, (f) CTL-M.
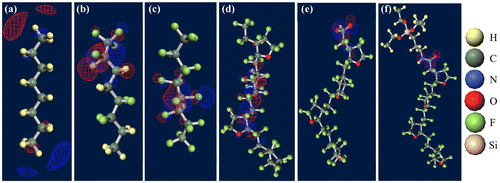
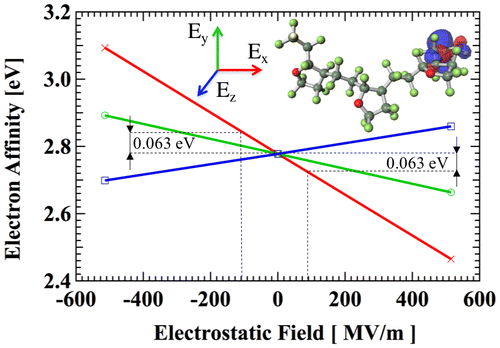
Figure 8. Strong electron-attracting characteristics of CTL-M end group.
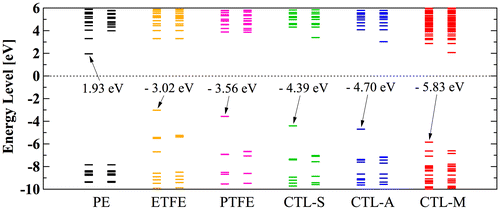
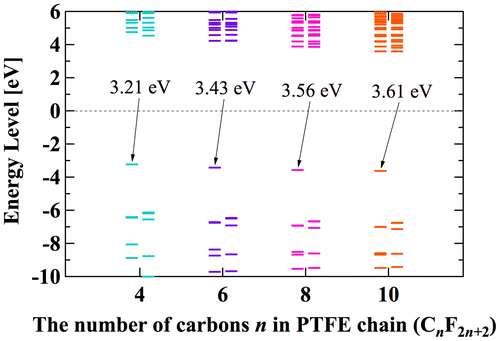
Figure 11. Calculated orbital energy level of polymer electrets.