Figures & data
Figure 1. XRD patterns of a mixture of the amorphous Sn(OH)4 powder and fumed silica for the Si/Sn molar ratio of 20; (a) before the mechanochemical treatment; (b) after grinding for 12 h, g(SiO2–SnO2)12; (c) after grinding for 24 h, g(SiO2–SnO2)24 (all patterns are vertically offset for clarity).
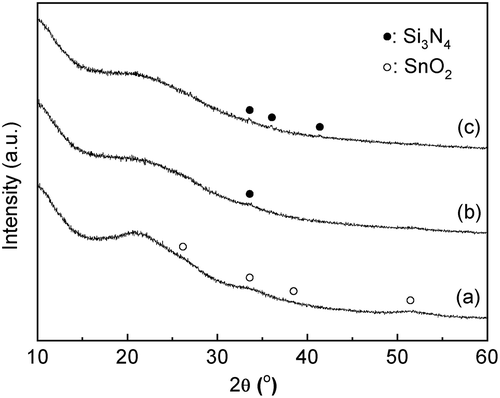
Figure 2. UV-vis spectra of a mixture of the amorphous Sn(OH)4 powder and fumed silica for the Si/Sn molar ratio of 20; (a) before the mechanochemical treatment; (b) after grinding for 12 h, g(SiO2–SnO2)12; (c) after grinding for 24 h, g(SiO2–SnO2)24.
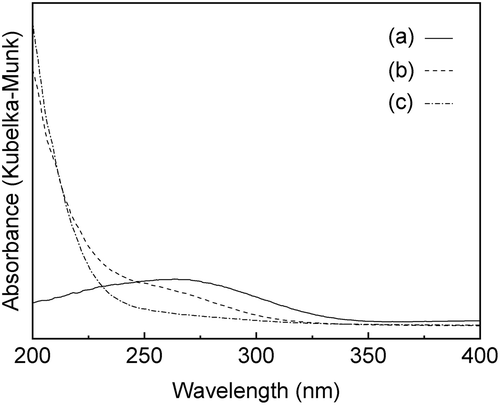
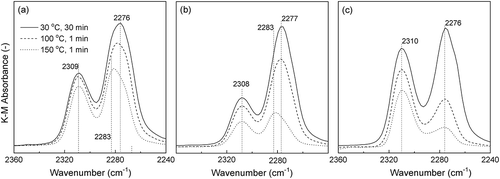
Figure 3. Sn 3d3/2 and 3d5/2 XPS spectra of (a) before the mechanochemical treatment; (b) after grinding for 24 h, g(SiO2–SnO2)24.
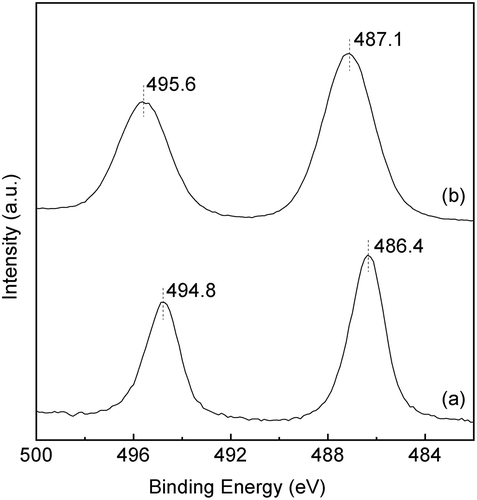
Figure 4. XRD patterns of Sn-MFI silicates Sn-SMC obtained by changing the HCl/Si molar ratio: (a) 0.20; (b) 0.25; (c) 0.30; (d) 0.35; (e) 0.36; (f) 0.37; (g) 0.38 (all patterns are vertically offset for clarity).
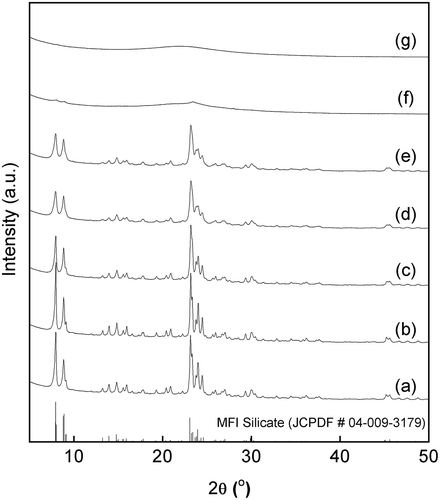
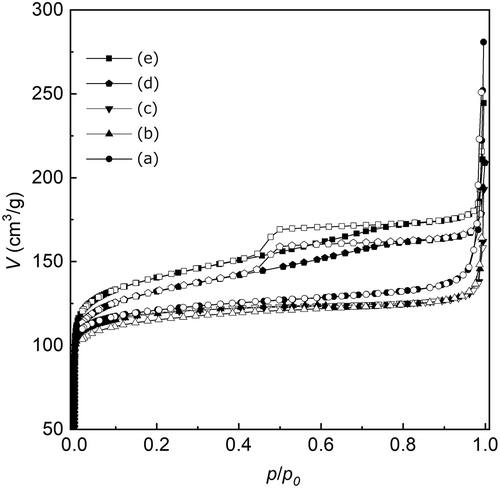
Figure 5. SEM images of Sn-MFI prepared with various HCl/Si molar ratios: (a) 0.20; (b) 0.25; (c) 0.30; (d) 0.35; (e) 0.36; (f) 0.37. The scale bar shown in (f) is common for all images. The insets are 2-fold magnified images for observing the surface roughness of the solid particles.
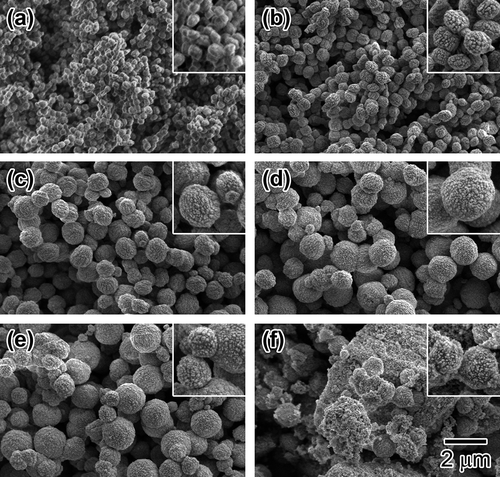
Figure 6. UV-vis spectra of Sn-MFI silicates Sn-SMC prepared at various HCl/Si molar ratios: (a) 0.20; (b) 0.25; (c) 0.30; (d) 0.35; (e) 0.36; (f) 0.37; (g) 0.38 (all spectra are vertically offset for clarity).
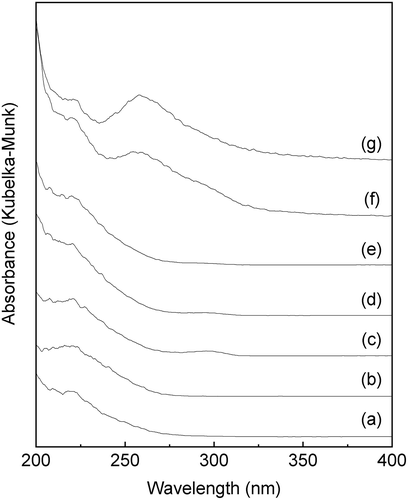
Figure 7. FTIR spectra of Sn-MFI silicates Sn-SMC obtained at various HCl/Si molar ratios: (a) 0.20; (b) 0.25; (c) 0.30; (d) 0.35; (e) 0.36 (all spectra are vertically offset for clarity).
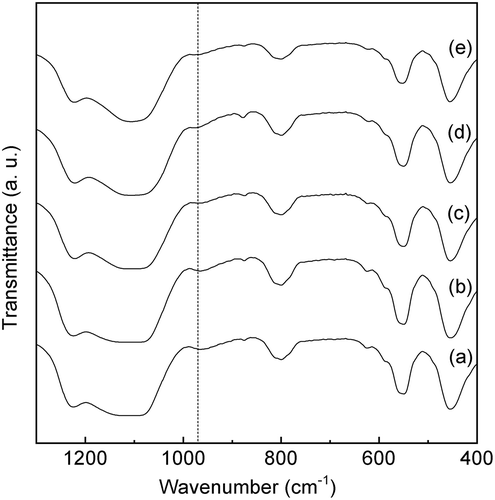
Table 1. The Sn content, BET surface area, and the external surface area of Sn-SMC.