Figures & data
Table 1. Structural difference between cocoon’s and spider’s silks
Table 2. Dominance of cocoon silk over spider silk
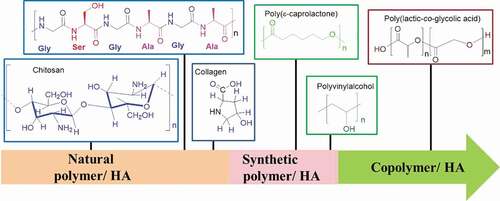
Table 3. Comparison of SF with other biomaterials
Table 4. Clinical applications of silk scaffolds
Table 5. Characteristics of hydroxyapatite obtained from mammalian sources
Table 6. Properties of HA obtained from fish sources
Table 7. Properties of HA from biogenic sources
Table 8. HA-based biomaterials in bone regeneration
![Figure 1. Publication frequency of SF and applications of SF as a biomaterial based on Scopus database: (a) SF-related publications, (b) SF used for tissue engineering, (c) SF used for bone tissue engineering, (d) SF/HA composites, and (e) to (h) remarkable studies related to parts (a) to (d) [Citation7]](/cms/asset/2457b6b8-4c3d-463e-8913-569a41e97bad/tsta_a_1748520_f0001_oc.jpg)
![Figure 2. Structure of silk fibroin [Citation18]](/cms/asset/d1ca81b6-b1aa-4cab-a090-34381b2259e7/tsta_a_1748520_f0002_oc.jpg)
![Figure 3. Mechanical strength comparison of silks obtained from the silkworm Bombyx mori drawn at different speeds [Citation29]](/cms/asset/4f9fd9be-09c5-41ad-8320-e795ba652e8d/tsta_a_1748520_f0003_oc.jpg)
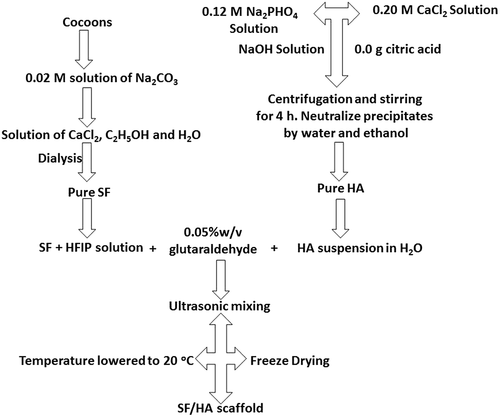
![Figure 4. Schematic representation of extraction of SF from cocoons [Citation41]](/cms/asset/ee121773-16b1-4d07-bda8-b567bdd26ffa/tsta_a_1748520_f0004_oc.jpg)
![Figure 5. Schematic representation of materials fabricated from silk fibroin: 4 days are required for complete extraction of silk fibroin, and different materials can be obtained at different intervals [Citation41]](/cms/asset/4bc1e20c-a0c7-45af-bc5e-9b43c9f51e5e/tsta_a_1748520_f0005_oc.jpg)
![Figure 6. (a) atomic structure of HA and (b) its projection along c-axis [Citation60]](/cms/asset/0e1f5ba5-178b-4f5a-adf5-4409fe661c21/tsta_a_1748520_f0006_oc.jpg)
![Figure 7. Typical transmission electron microscopy images of mesoporous HA calcined at 600 ° C: (a) 100 nm scale and (b) 20 nm scale [Citation109]](/cms/asset/292981ac-89de-4ba9-ab51-26d9599979a7/tsta_a_1748520_f0007_b.gif)
![Figure 8. FE-SEM images of calcium phosphate nano-particles. (a–c) Nano-particles synthesized at 40 C: (a) 10% F127; (b) 40% F127; and (c) 80% F127. (d–f) Nano-particles synthesized at 100 C: (d) 10% F127; (e) 40% F127; and (f) 80% F127. Magnification is 80,000 times [Citation108]](/cms/asset/cf35869a-6187-43db-a7ab-e87495778809/tsta_a_1748520_f0008_b.gif)
![Figure 11. FE-SEM images of pure SF after three cycles of Ca-P treatment and mineralized SF/HA nanofibres: (a) pure silk nanofibres and (b–d) SF/HA nanofibres with different magnification [Citation40]](/cms/asset/c5aa20ee-d9c1-43cc-b20c-6f92513dc8e7/tsta_a_1748520_f0011_b.gif)
![Figure 12. FTIR spectra of SF/HA porous scaffolds with different molar% contents of nHA: (a) 0%, (b) 10%, (c) 30%, (d) 60%, (e) 70%, and (f) 100% [Citation184]](/cms/asset/abab86df-0a22-4adc-8b4f-c6299d6ef7d3/tsta_a_1748520_f0012_b.gif)
![Figure 13. Elemental analysis of calcium (Ca), phosphorus (P), with oxygen (O) and nitrogen (N) in (a–d) Increase in SF/HA concentration is confirmed via EDS mapping of Ca (e–g) [Citation185]](/cms/asset/a873ba9d-a002-4ca7-9d82-96340e238fe0/tsta_a_1748520_f0013_oc.jpg)
![Figure 14. XRD patterns of different scaffolds of SF/HA composites with (a) HA0, (b) HA10, (c) HA30,(d) HA60, and (e) HA70 [Citation184].2θ (degree)](/cms/asset/d1bb3346-ff30-4b3f-8e0e-6d1a3cab6a99/tsta_a_1748520_f0014_b.gif)
![Figure 15. (a) Water contact angle of SF/HA scaffolds with different HA contents (p < 0.05) and (b) photographs of water droplets on SF/HA scaffolds [Citation185]](/cms/asset/6ff18a45-988b-473a-bfa8-f8f0a8962ea8/tsta_a_1748520_f0015_oc.jpg)
![Figure 16. Thermogravimetric curves of nHA, pure SF and SF/HA (molar ratio = 8:2) [Citation40]](/cms/asset/0b6708ba-75ab-4c23-8eed-0f5363da908d/tsta_a_1748520_f0016_b.gif)
![Figure 17. SEM images of porous SF/HA scaffolds with different molar contents of nHA: (a) 10% (b) 0% (c) 10% (d) 30% (e) 60% and (f) 70% [Citation184]](/cms/asset/00153967-3dd4-48c3-84ff-23cb052f6b49/tsta_a_1748520_f0017_b.gif)
![Figure 18. Cell proliferation investigation of rBMSCs cultured on 30% SF/HA (dark in colour) and pure HA (light in colour) [Citation185]](/cms/asset/d3460884-8943-471d-973e-c86c0456280b/tsta_a_1748520_f0018_oc.jpg)
![Figure 19. SEM images of attachment and proliferation of rBMSCs cultured on SF/HA scaffolds for 7, 14 and 21 days (a,c, e, g, i and k) scale bars = 100 µm; (b, d, f, h, j and l) scale bars = 50 µm [Citation185]](/cms/asset/56c2c55f-0c66-4e68-842c-b2d549828724/tsta_a_1748520_f0019_b.gif)
![Figure 20. Proliferation activities of cells cultured on pure SF, SF/nHA, and tissue culture dish (TCD) from 3 to 14 days. (p ≥ 0.05) indicates significant increase in proliferation activity [Citation40]](/cms/asset/39fb9392-f11a-493c-a637-b66fa124f72b/tsta_a_1748520_f0020_oc.jpg)
![Figure 21. ALP activity. Osteogenic differentiation of (a) rBMSCs on SF/HA scaffolds with 0 and 30 mol% HA, and (b) MC3TC-E1 on pure and mineralized SF/nHA after 5, 7, 10 and 14 days. ⋆ shows difference in ALP activity [Citation40,Citation185]](/cms/asset/cb94d29b-4986-476e-9c91-1bdd391c4b38/tsta_a_1748520_f0021_oc.jpg)
![Figure 22. (a–d) In vivo biocompatibility of HA/SF-5% (black arrows denote lymphocytes, eosin and haematoxylin staining). (e–h) Increment of lymphocytes at the implantation site between control and experimental groups [Citation187]](/cms/asset/3947b3e9-d5c7-4833-8f0d-2896b53f2baf/tsta_a_1748520_f0022_oc.jpg)
![Figure 23. Photographs of rabbit bones on 12th week showing (a) no new bone formation without SF/HA-3, (b) new bone formation at bone defect site with SF/HA-3, and (c) new cortical bone formation and remodelling site with SF/HA-3 consisting BMSCs [Citation188]](/cms/asset/a5993a67-27d7-460c-ab4d-6419ccecbbde/tsta_a_1748520_f0023_b.gif)
![Figure 24. Radiographic illustration of gross anatomy of transplanted bone: structure of the defective radius at 12 weeks after operation in the experimental group (a) and the control group (b). Arrow indicates the transplantation site [189]](/cms/asset/f8479b2e-3120-4326-b708-b7601dc61911/tsta_a_1748520_f0024_oc.jpg)