Figures & data

Figure 2. Electrical characteristic of paper-based metal electrodes without bending obtained in wireless manner. (a) Change in interfacial potential with pH from 4.01 to 9.18 using the paper-based Au electrode with the Ta2O5 film. (b) Calibration curve for pH sensitivity based on (a). The number of measurements was five. (c) Change in interfacial potential with Na+ concentration from 5 to 500 mM detected using the paper-based Au electrode with the FPS-based Na+-sensitive membrane. (d) Calibration curve for pNa sensitivity based on (c). The number of measurements was five. (e) pNa response of the paper-based Au electrode with the FPS-based Na+-sensitive membrane in a buffer with 100 mM K+. In (a) and (b), the interfacial potentials at pH 9.18 were offset to zero. In (c) and (d), the interfacial potentials at 5 mM Na+ were offset to zero. In (e), the interfacial potential at 5 mM Na+ and 100 mM K+ was offset to zero.
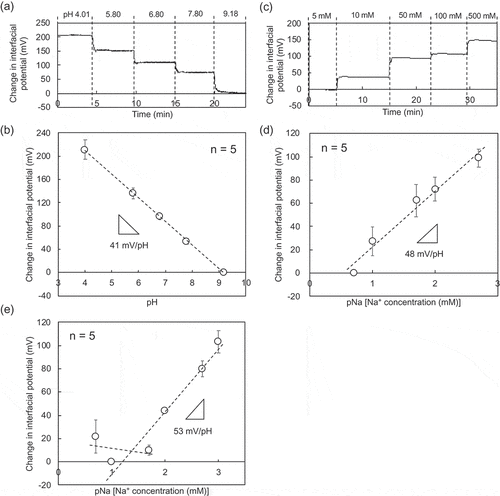
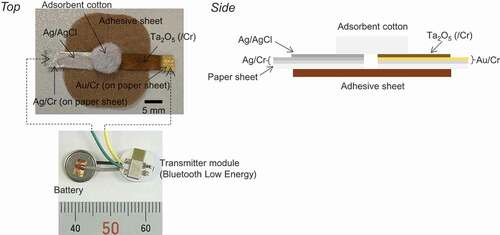
Figure 3. Electrical characteristic of paper-based metal electrode with bending obtained in wireless manner. (a) Photograph of polyvinylchloride resin-based cylinders with different radii (r) from 6.5 to 25 mm. The paper-based Au electrode with the FPS-based Na+-sensitive membrane, which was used in (b), (c), and (d), was rolled around the cylinder with the smallest radius of 6.5 mm (upper-right photograph). (b) Real-time monitoring of μVout using the paper-based Au electrode with the FPS-based Na+-sensitive membrane, which was rolled around the cylinder with the smallest radius of 6.5 mm (at useful life 0 week). (c) Useful life of the paper-based Au electrode with the FPS-based Na+-sensitive membrane, which was rolled around the cylinder with the smallest radius of 6.5 mm. The change in pNa sensitivity was evaluated for 0 to 4 weeks. The number of measurements performed at each time was three. (d) Change in pNa sensitivity with radius of curvature from 6.5 to 25 mm detected using the paper-based Au electrodes with the FPS-based Na+-sensitive membrane, which were rolled around each cylinder.
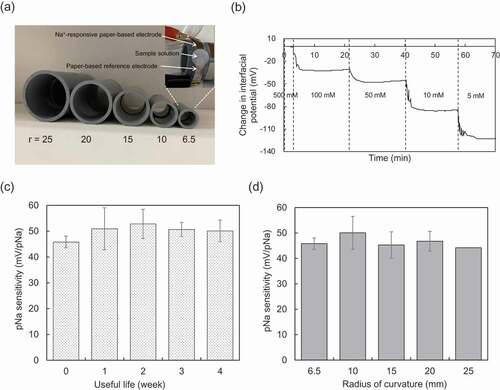
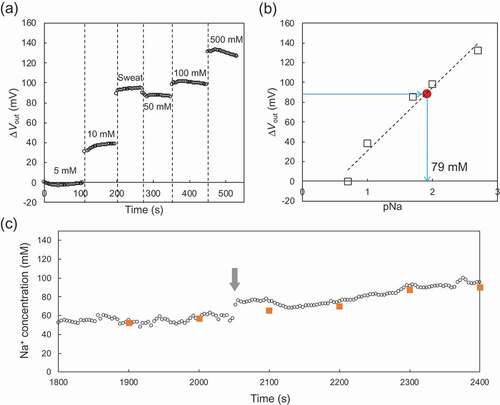
Figure 4. In vitro real-time monitoring of μVout for sweat sample using the paper-based Au electrode with the FPS-based Na+-sensitive membrane, which was rolled around the cylinder with the smallest radius of 6.5 mm. (a) μVout with changing Na+ concentrations from 5 mM to 500 mM in the buffer. The sweat sample was added after the measurement at 10 mM. (b) Calibration curve for pNa sensitivity based on (a). The Na+ concentration in the sweat sample was calculated from the calibration curve. (c) Changes in Na+ concentration determined by on-body measurement. The Na+ concentrations were calculated from the calibration curve obtained in (a) and (b). The Na+ concentration gradually increased after the time indicated by the arrow.