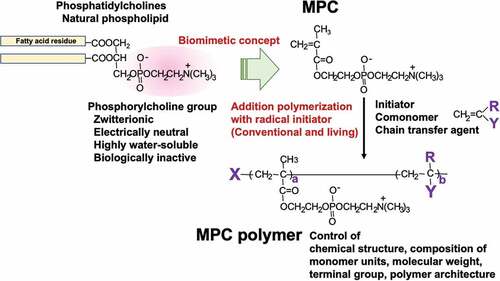
Figures & data
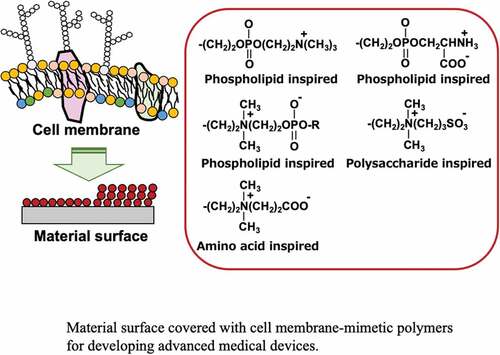
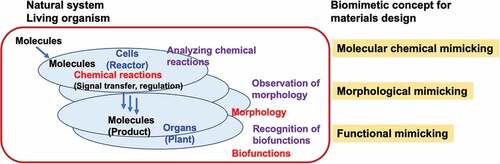
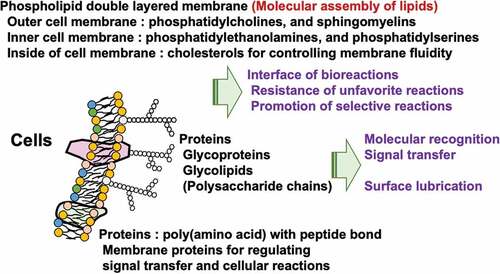
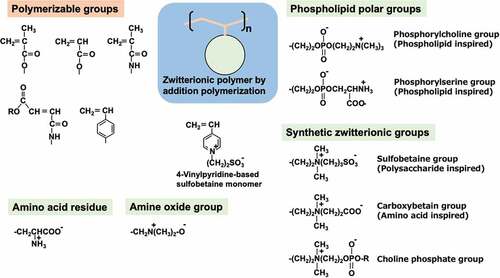
Figure 1. Representative biological reactions of medical devices and their required surface properties.
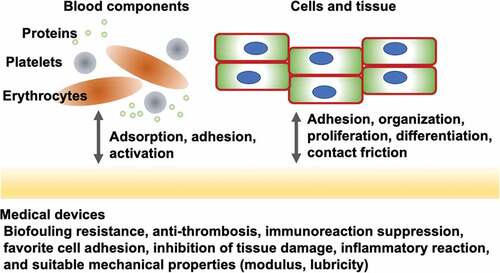
Figure 6. (a) Schematic representation of hydration state of PMPC and (b) molecular simulation image of PMPC chain in aqueous medium.
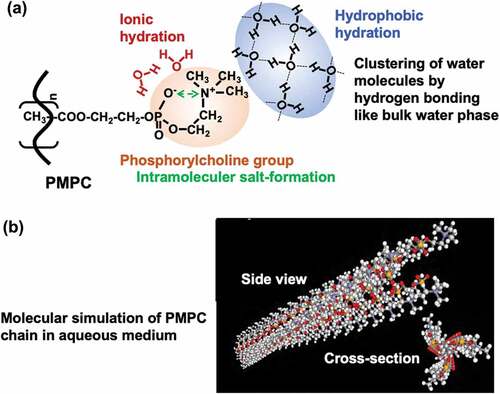
Figure 7. Biomimetic surface immobilization procedure with zwitterionic polymers. (a) MPC polymer with catechol moiety prepared by polymer reaction and the atom force micrographic images at the surfaces titanium substrate before and after surface treatment. Reprinted with permission from [Citation103]. Copyright (2011) elsevier. (b) Copolymerization of MPC with dopamine methacrylamide (DMA). Reprinted with permission from [Citation104]. Copyright (2019) American chemical society. (c) Coating procedure on the substrate by polymer reaction with MPC polymer with the epoxy group following dopamine polymerization. Reprinted with permission from [Citation105]. Copyright (2022) elsevier. (d) Surface-initiated atom transfer radical polymerization (SI-ATRP) after immobilization of the initiator by dopamine chemistry. Reprinted with permission from [Citation106]. Copyright (2015) American chemical society.
![Figure 7. Biomimetic surface immobilization procedure with zwitterionic polymers. (a) MPC polymer with catechol moiety prepared by polymer reaction and the atom force micrographic images at the surfaces titanium substrate before and after surface treatment. Reprinted with permission from [Citation103]. Copyright (2011) elsevier. (b) Copolymerization of MPC with dopamine methacrylamide (DMA). Reprinted with permission from [Citation104]. Copyright (2019) American chemical society. (c) Coating procedure on the substrate by polymer reaction with MPC polymer with the epoxy group following dopamine polymerization. Reprinted with permission from [Citation105]. Copyright (2022) elsevier. (d) Surface-initiated atom transfer radical polymerization (SI-ATRP) after immobilization of the initiator by dopamine chemistry. Reprinted with permission from [Citation106]. Copyright (2015) American chemical society.](/cms/asset/f530152b-bb0e-4dd8-b818-0d1fb8928485/tsta_a_2119883_f0007_oc.jpg)
Table 1. List of medical devices using biomimetic zwitterionic polymers.
Figure 8. (a) Chemical structure of cross-linkable MPC polymer. (b) Morphology of cardiovascular stent treated with the MPC polymer and (c) fluorescence image of it after staining with rhodamine 6G. Reprinted with permission from [Citation140]. Copyright (2005) Wiley. (d) in vitro blood coagulation test of a cerebrovascular stent using blood circuit method and (e) SEM image of the stent without and with MPC polymer treatment after 60-min contact with whole blood. (f) the concentration of thrombin-antithrombin complex (TAT) in blood after 60-min contact with whole blood. (d), (e), (f) are reprinted with permission from [Citation141]. Copyright (2005) Wiley.
![Figure 8. (a) Chemical structure of cross-linkable MPC polymer. (b) Morphology of cardiovascular stent treated with the MPC polymer and (c) fluorescence image of it after staining with rhodamine 6G. Reprinted with permission from [Citation140]. Copyright (2005) Wiley. (d) in vitro blood coagulation test of a cerebrovascular stent using blood circuit method and (e) SEM image of the stent without and with MPC polymer treatment after 60-min contact with whole blood. (f) the concentration of thrombin-antithrombin complex (TAT) in blood after 60-min contact with whole blood. (d), (e), (f) are reprinted with permission from [Citation141]. Copyright (2005) Wiley.](/cms/asset/f9d77a1d-fe86-474f-ba2b-6a752fe8c656/tsta_a_2119883_f0008_oc.jpg)
Figure 9. (a) Procedure of MPC polymer (PMBA) nanofiber fabrication by electrospinning deposition (ESD) method and following photoirradiation. Chemical structure of photoreactive PMBA and SEM image of nanofiber membrane are indicated. Reprinted with permission from [Citation174]. Copyright (2017) American chemical society. (b) Surface treatment procedure on polypropylene non-woven membrane with chemically cross-linkable sulfobetaine polymer, poly(gma-co-SBMA). Reprinted with permission from [Citation175]. Copyright (2019) elsevier.
![Figure 9. (a) Procedure of MPC polymer (PMBA) nanofiber fabrication by electrospinning deposition (ESD) method and following photoirradiation. Chemical structure of photoreactive PMBA and SEM image of nanofiber membrane are indicated. Reprinted with permission from [Citation174]. Copyright (2017) American chemical society. (b) Surface treatment procedure on polypropylene non-woven membrane with chemically cross-linkable sulfobetaine polymer, poly(gma-co-SBMA). Reprinted with permission from [Citation175]. Copyright (2019) elsevier.](/cms/asset/37cb0b6a-abdd-4d8c-8ae1-e184347849eb/tsta_a_2119883_f0009_oc.jpg)
Figure 10. Surface treatment on blood circuit devices. (a) Coating procedure and platelet adhesion examined on PVC treated with MPC polymer (PMD). Reprinted with permission from [Citation181]. Copyright (1994) Walters Kluwer. (b) Photoreaction of carboxybetaine polymer with photoreactive benzophenone group (PCB) on plasticized PVC and reduction of elution of plasticizer by PCB treatment. Reprinted with permission from [Citation135]. Copyright (2020) American chemical society.
![Figure 10. Surface treatment on blood circuit devices. (a) Coating procedure and platelet adhesion examined on PVC treated with MPC polymer (PMD). Reprinted with permission from [Citation181]. Copyright (1994) Walters Kluwer. (b) Photoreaction of carboxybetaine polymer with photoreactive benzophenone group (PCB) on plasticized PVC and reduction of elution of plasticizer by PCB treatment. Reprinted with permission from [Citation135]. Copyright (2020) American chemical society.](/cms/asset/c17ef463-186d-4ac0-9d32-44c1a1420bc7/tsta_a_2119883_f0010_oc.jpg)
Figure 11. (a) Schematic representation of the surface structure of natural cartilage. Reprinted with permission from [Citation194]. Copyright (2021) Wiley. (b) Photoinduced grafting of PMPC on cross-linked UHMWPE and transmission electron microscope image at the grafting surface. (c) Relationship between static water-contact angle and dynamic friction coefficient of PMPC grafted cross-linked UHMWPE surface. (b) and (c) are reprinted with permission from [Citation195]. Copyright (2008) Wiley. (d) X-ray photo images of PMPC-grafted cross-linked UHMWPE liner installed artificial hip joint implanted into the patient during various implant periods. Reprinted with permission from [Citation196]. Copyright (2017) Wiley.
![Figure 11. (a) Schematic representation of the surface structure of natural cartilage. Reprinted with permission from [Citation194]. Copyright (2021) Wiley. (b) Photoinduced grafting of PMPC on cross-linked UHMWPE and transmission electron microscope image at the grafting surface. (c) Relationship between static water-contact angle and dynamic friction coefficient of PMPC grafted cross-linked UHMWPE surface. (b) and (c) are reprinted with permission from [Citation195]. Copyright (2008) Wiley. (d) X-ray photo images of PMPC-grafted cross-linked UHMWPE liner installed artificial hip joint implanted into the patient during various implant periods. Reprinted with permission from [Citation196]. Copyright (2017) Wiley.](/cms/asset/ea6f1cfa-f6bd-4bdd-926a-7abcfca35e54/tsta_a_2119883_f0011_oc.jpg)
Figure 12. Various stable lubricious surfaces using zwitterionic polymers. (a) PMPC interpenetrating network system. Reprinted with permission from [Citation198]. Copyright (2018) elsevier. (b) Photoreactive cross-linking system to enhance the stability of lubrication layer. Reprinted with permission from [Citation213]. Copyright (2020) American chemical society. (c) a particle-based system using silica nanoparticles covered with MPC grafting layer as lubricant and drug reservoir. Reprinted with permission from [Citation214]. Copyright (2020) elsevier. (d) Bioconjugate system composed of natural biomolecule chitosan and PMPC grafting. Reprinted with permission from [Citation215]. Copyright (2022) Wiley.
![Figure 12. Various stable lubricious surfaces using zwitterionic polymers. (a) PMPC interpenetrating network system. Reprinted with permission from [Citation198]. Copyright (2018) elsevier. (b) Photoreactive cross-linking system to enhance the stability of lubrication layer. Reprinted with permission from [Citation213]. Copyright (2020) American chemical society. (c) a particle-based system using silica nanoparticles covered with MPC grafting layer as lubricant and drug reservoir. Reprinted with permission from [Citation214]. Copyright (2020) elsevier. (d) Bioconjugate system composed of natural biomolecule chitosan and PMPC grafting. Reprinted with permission from [Citation215]. Copyright (2022) Wiley.](/cms/asset/45c3bbdd-1f3b-4089-b257-2215d0447371/tsta_a_2119883_f0012_oc.jpg)
Figure 13. (a) Microstructure of surface of the conjunctiva epithelium observed with a transmission electron microscope. (b) Schematic representation of biomolecules at the surface of the conjunctiva epithelium. Reprinted with permission from [Citation230]. Copyright (2003) elsevier.
![Figure 13. (a) Microstructure of surface of the conjunctiva epithelium observed with a transmission electron microscope. (b) Schematic representation of biomolecules at the surface of the conjunctiva epithelium. Reprinted with permission from [Citation230]. Copyright (2003) elsevier.](/cms/asset/cf53f9e0-cc6a-4437-a570-2693144ad293/tsta_a_2119883_f0013_oc.jpg)
Figure 14. (a) Chemical structure of silicone hydrogel contact lens grafted with the MPC polymer (b) Cross-sectional view of silicone hydrogel contact lens grafted with the MPC polymer. Reprinted with permission from [Citation233]. Copyright (2021) elsevier. (c) Surface elastic properties of silicone hydrogel contact lens and natural corneal tissue. Transmission electron microscopy images (a), (b), and (c) correspond to lettering in the bar graph. Reprinted with permission from [Citation132]. Copyright (2021) American chemical society.
![Figure 14. (a) Chemical structure of silicone hydrogel contact lens grafted with the MPC polymer (b) Cross-sectional view of silicone hydrogel contact lens grafted with the MPC polymer. Reprinted with permission from [Citation233]. Copyright (2021) elsevier. (c) Surface elastic properties of silicone hydrogel contact lens and natural corneal tissue. Transmission electron microscopy images (a), (b), and (c) correspond to lettering in the bar graph. Reprinted with permission from [Citation132]. Copyright (2021) American chemical society.](/cms/asset/cced581a-9554-4126-b3a5-94efea18dace/tsta_a_2119883_f0014_oc.jpg)