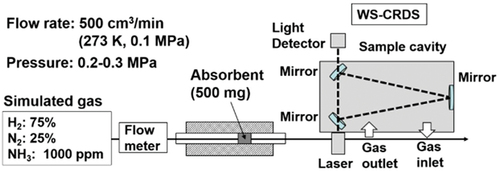
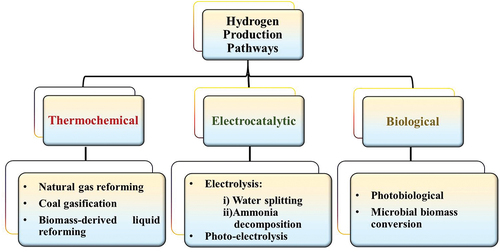
Figures & data
Figure 1. (a) Energy density of a range of chemical fuels. (b) Estimated rise in annual ammonia production.
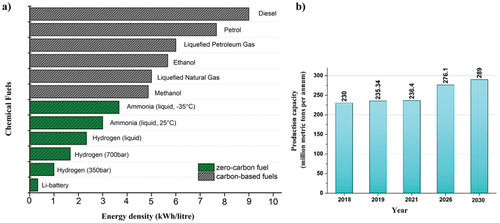
Table 1. Fuel-associated ammonia technologies.
Figure 2. Estimated net zero carbon energy goals from 2020 till the end of the year 2050 (citation: 2020, International Energy Agency (IEA)).
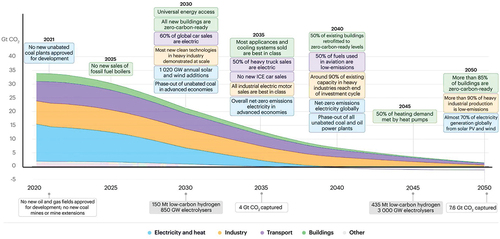
Table 2. Summary of the basic conditions for H2 production through non-renewable sources.
Table 3. Different hydrogen production technologies from a renewable source of water.
Figure 4. Catalytic activity for ammonia decomposition over alkaline earth metals modified Ni/Y2O3; (a) TEM images, (b) particle size distribution, (c) NH3 conversion percentage, and (d) stability performance of SrO-Ni/Y2O3 (5:40 wt%), (copyright @ 2016 RSC Advances).
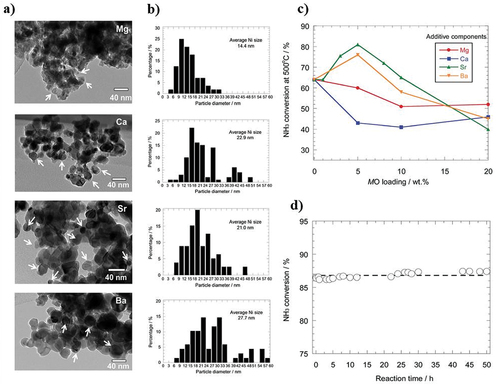
Figure 5. Incorporation of catalytic membrane for ADR process. (a) Schematic catalytic membrane reactor, (b) catalytic activities, (c) TEM image of bimodal, (d) TEM image of monomodal, and e) SEM image of bimodal catalytic membrane (copyright @ 2011 Elsevier). (f) Mechanistic interaction of Ru doping in LaxCe1-xOy composites and (g) catalytic performance by varying different temperature for NH3 conversion (copyright @ 2021 Elsevier).
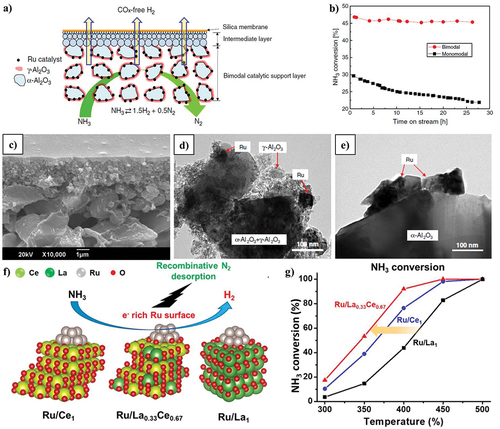
Table 4. Catalysts based on noble metals utilization to decompose ammonia.
Figure 6. Varying different temperature for NH3 conversion over iron-based nanostructured composites (a) 1st catalytic performance, (b) 2nd catalytic performance, and (c) stability performance (copyright @ 2015 Elsevier). Varying different temperature for NH3 conversion over ceria-based oxide supported nickel composites (d) catalytic performance, (e) Arrhenius plot, and (f) stability performance (copyright @ 2020 Elsevier). (g) Varying different temperature for NH3 conversion over ceria-based oxide supported Ni, Ru, and Ni-Ru composites (copyright @ 2019 Elsevier), varying different temperature for NH3 conversion over ceria-based oxide supported Ru/Al2O3 composites. (h) Comparative study with literature reports and (i) catalytic performance (copyright @ 2023 Elsevier).
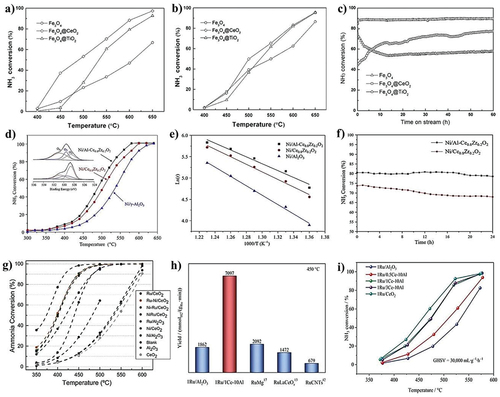
Figure 7. NH3 decomposition by (a) ion exchange method, (b) NaOH deposition method, (c) TEM images of 1. 10CoTi-NT 2. 10CoNaTi-NT (copyright @ 2019 Elsevier). (d) Synthesis scheme of MoS2 catalysts, (e) catalytic activity of MoS2 composites, and (f) stability performance (copyright @ 2020 Elsevier).
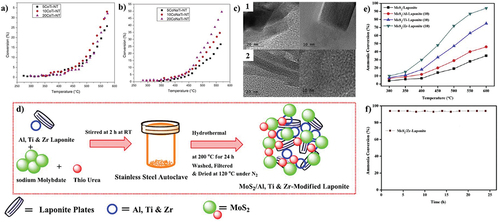
Table 5. Efficiency of reported non-noble metal catalysts for ammonia-decomposition.
Table 6. Catalytic activity of metallic amides, imides, carbides, and nitrides.
Figure 8. Ammonia decomposition over bimetallic Ni- and Co-based catalyst (copyright @ 2019 Elsevier).
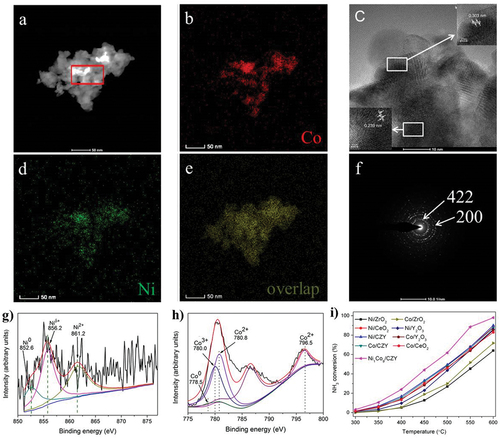
Figure 9. Schematic illustration of PEM fuel cell for H2 production from ammonia (copyright @ 2018 Elsevier).