Figures & data
Figure 1. Panel of assays to measure (in vitro) ligand binding and effector function of Enbrel /GP2015 in relation to the structure of the etanercept molecule.
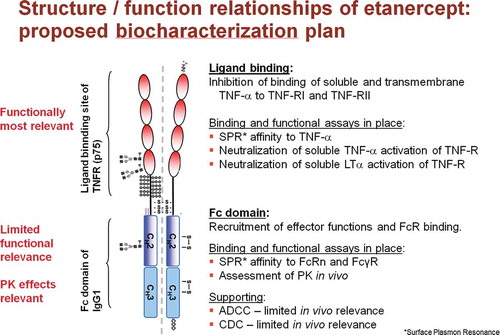
Table 1. The experimental groups in the human TNF transgenic mouse model Tg197 [Citation24] used to compare the therapeutic efficacy of GP2015 and Enbrel at a pharmacologically sensitive dose of 10 mg/kg.
Table 2. GP2015/Enbrel neutralization of TNF-α and LT-α in an RGA, binding to TNF-α and Fc receptors (SPR; Biacore assay).
Table 3. Ratios of mean PK parameters in male rabbits for five GP2015 formulations relative to Enbrel/EU [25 mM phosphate/25 mM arginine HCl buffer].
Figure 2. Dose-dependent inhibition of TNF-α function by GP2015 and Enbrel in a cellular NFκB-dependent luciferase reporter gene assay.
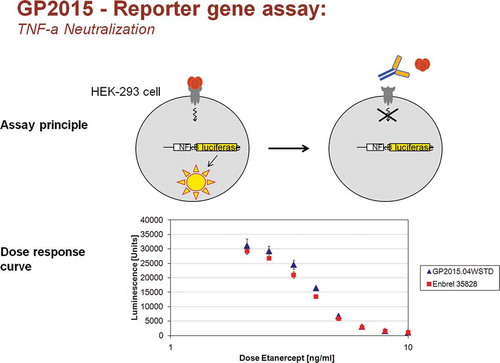
Figure 3. Results for TNF-α neutralization for different batches of Enbrel (Originator) and GP2015 (Biosimilar) in the reporter-gene assay.
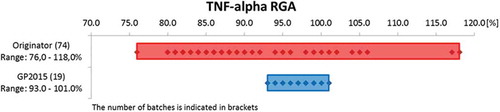
Figure 4. Comparative pharmacokinetic profiles after single 8 mg/kg bodyweight (b.w.) subcutaneous (s.c.) administration to rabbits.
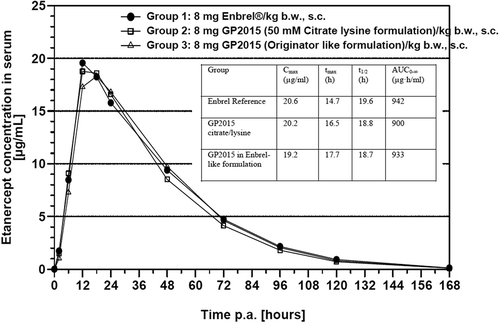
Figure 5. Arthritic score assessment in the human TNF transgenic mouse model Tg197 [Citation25]. At a pharmacologically sensitive dose of 10 mg/kg, GP2015 and Enbrel were indistinguishable in inhibiting arthritic disease symptoms when compared to vehicle buffer-treated control group.
![Figure 5. Arthritic score assessment in the human TNF transgenic mouse model Tg197 [Citation25]. At a pharmacologically sensitive dose of 10 mg/kg, GP2015 and Enbrel were indistinguishable in inhibiting arthritic disease symptoms when compared to vehicle buffer-treated control group.](/cms/asset/c629e858-141f-4d2b-b03c-336d0603c634/iebt_a_1217329_f0005_oc.jpg)
Figure 6. The histopathology and in-life mean arthritic score at week 10 in experimental Tg197 mice. By week 10 the mean disease severity scores of the biweekly treated groups (from week 6 onwards), were as follows: (G5) Enbrel at 10 mg/kg = 3.13 (HS) and 1.04 (AS), (G6) GP2015 at 10 mg/kg = 2.89 (HS) and 0.9 (AS), (G7) vehicle GP2015 buffer = 3.58 (HS) and 1.43 (AS), (G8) Enbrel at 30 mg/kg = 1.53 (HS) and 0.61 (AS) Control mice at week 6 had a score of 2.81 (HS) and 1.19 (AS). Bars indicate standard errors of the mean.
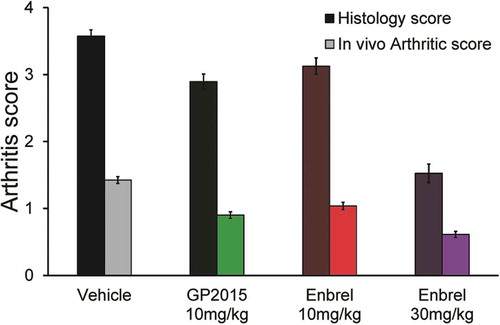
Table 4. Mean ± standard deviation toxicokinetic parameters following subcutaneous administration of GP2015 or Enbrel 15 mg/kg subcutaneously (once every 3 days for 4 weeks) to groups of cynomolgus monkeys (n = 3 in each group).
