Figures & data
Figure 1. The analytical capability of mass spectrometry. (a) The detection of a specific host cell protein at 1 ppm using a modern electrospray ionization source mass spectrometer. The extracted ion chromatograms of three fragment ions used for characterization and quantitation are shown in green, orange and blue. The doted lines indicate the range for integration and relative quantitation. (b) Analysis of polyethylene glycol (PEG)-filgrastim using a matrix assisted light desorption (MALDI) source mass spectrometer in the 1990s (upper panel) and analysis of PEG-filgrastim using a modern electrospray ionization source mass spectrometer showing resolution of individual PEG extension units and associated variants (lower panel). A representative model of PEG-filgrastim shows the protein therapeutic component and its associated PEG chain.
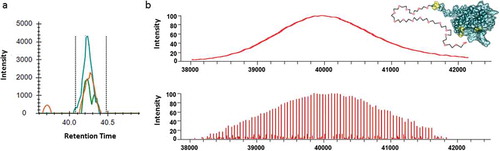
Figure 2. Strategic overview of continued process verification. Continued process verification helps to ensure process consistency by allowing fast and objective identification of outliers or trends in production data. Statistical process control limits, in general three standard deviations above the mean (3s) are used to define out of expectation (OOE) data, allowing preventive or corrective action to be taken before outlies approach specification limits and are out of specification (OOS). Specification limits are set based clinical data and manufacturing capability and prevent the release of batches with differences in safety or efficacy.

Figure 3. Strategic overview for the generation of a two tiered reference standard. A strategic overview for the generation and maintenance of a two tiered reference standard over the course of biopharmaceutical development to commercialization. A large scale phase III or pivotal trial drug substance batch produced under cGMP conditions is used for generating the in house primary reference standard. All working standard batches used to release commercial material must first be compared to the primary reference and comply with primary reference standard specifications prior to use, ensuring a consistent standard throughout the lifecycle of the product.
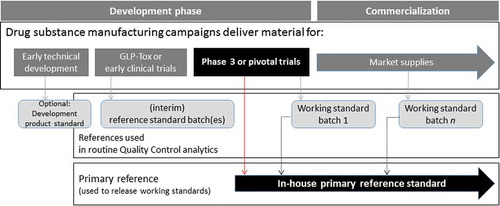
Figure 4. Demonstration of comparability following manufacturing changes allows maintenance of clinical performance. An example diagram showing the theoretical impact of manufacturing changes on a measurable property of a biosimilar product and reference product (Ref.) over time. While multiple changes over time may result in divergent abundance of a measurable property, the comparability exercise establishes that there are no clinically meaningful difference in the products and allows bridging to the original safety and efficacy established upon product approval. It should be noted that the concept illustrated in this Figure applies equally to the life-cycle management of originator and biosimilar drugs as well as to products known in the US as ‘interchangeable biologics’ (biosimilars that can be substituted by a pharmacist without first obtaining approval from the prescribing physician).
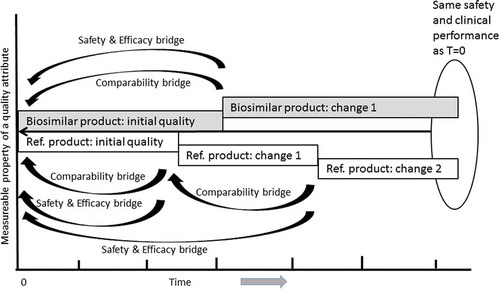
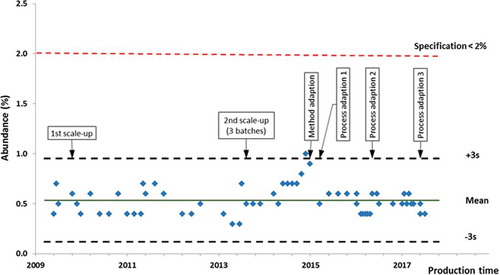
Figure 5. Long term large scale production data for a representative product variant in filgrastim.
The most abundant single product variant is a critical quality attribute describing multiple process sensitive variants in filgrastim detected using a single reverse phase chromatography method. The relative amount of the most abundant variant in a batch is reported. The most abundant single product variant batch release values from the Sandoz filgrastim biosimlar *Zarzio® are reported from 2009 to 2017. Process control limits of three standard deviations above the mean (3s) are indicated by dashed black lines and the mean is indicated by a green line. The release specification of less than 2.0% is indicated by dashed red line. Manufacturing up-scales and process adaptions are specified.
*Zarzio® (filgrastim) was licensed in the European Union in 2009.
The same drug was approved in the US in 2015 as Zarxio® (filgrastim-sndz).