Figures & data
Table 1. Patient demographics and disease characteristics at baseline.
Figure 1. Proportion of patients achieving a 75% reduction in Psoriasis Area Severity Index (PASI) scores (PASI75), a 90% reduction in PASI (PASI90) and a 100% reduction in PASI (PASI100) during the 52 weeks of secukinumab treatment.
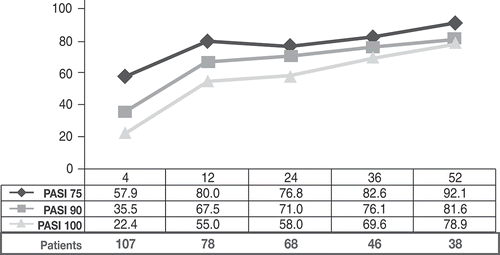
Figure 2. Proportion of patients naïve to biological therapies (naïve) and patients with prior exposure to biologics (PBT) achieving (a) 75% reduction in Psoriasis Area Severity Index (PASI) scores (PASI75), (b) 90% reduction in PASI (PASI90), and (c) 100% reduction in PASI (PASI100) during the 52 weeks of treatment with secukinumab.
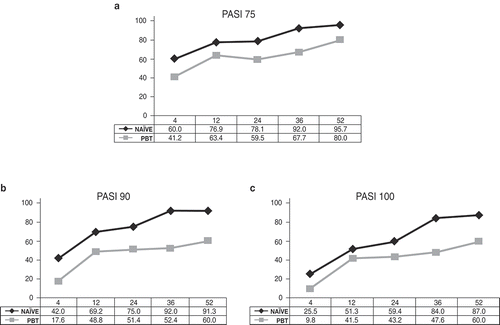
Table 2. Univariate logistic regression analysis on PASI75, PASI90, and PASI100 response.
Figure 3. Proportion of patients achieving (a) 75% reduction in Psoriasis Area Severity Index (PASI) scores (PASI75), (b) 90% reduction in PASI (PASI90), and (c) 100% reduction in PASI (PASI100) during the 52 weeks of secukinumab treatment, according to the number of previous biological therapies (PBT).
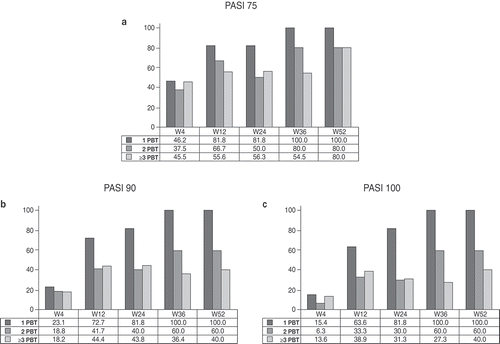
Table 3. Adverse events during secukinumab exposure period.
