Figures & data
Figure 1. Schematic of main cell types in the lung and liver.
(a) The lung can roughly be divided into three compartments proximally to distally: the trachea, bronchioles and alveoli. Ciliated cells are mainly present in the trachea and bronchioles, and are the key target cell for gene therapy for primary ciliary dyskinesia. For CF, the ciliated cells primarily lining the bronchioles are targeted. Basal cells represent one of the purported stem cell niches and are therefore an important target for gene therapy. The location of basal cells below the ciliated and club cells of the stratified epithelium, means that some form of mechanical or chemical cell junction disruption is required to access them. The alveolar type 2 (AT-2) cells are the main producers of surfactant proteins and thus a target cell type for gene therapy for surfactant deficiencies. (b) In the liver, the predominant target cell is the hepatocyte, which are the main source of many proteins in the blood such as albumin. Hence, they constitute an important target for the production of secreted proteins such as alpha-1 antitrypsin and clotting factors VIII and IX. Both Kupffer cells and stellate cells have reported antigen presentation capacity, which suggests that transduction and expression in such cells is to be avoided to prevent unnecessary immune responses to transgene product or viral proteins. (c) Location of viral receptors on polarized cells of the lung epithelium can affect in-vivo applications. The receptor for the lentiviral F/HN pseudotype is directly accessible via the apical surface, whereas receptors for VSV-G and GP64 pseudotypes may be located more basolaterally, requiring the addition of chemical adjuvants for efficient airway gene delivery. A similar strategy is required to access the basal cells of the epithelium.
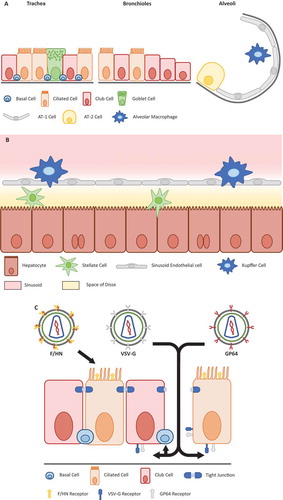
Figure 2. AAV capsid evolution and lentivirus pseudotyping.
(a) Directed viral capsid evolution is commonly used to engineer novel capsid variants that are efficient in the transduction of a very specific cell type or tissue. To achieve this, a library of serotypes are used, which could consist of already known serotypes, or novel ones containing random parts of other serotypes (capsid shuffling), or capsids derived by error-prone PCR amplification that usually results in one to three point mutations (1). These libraries are used to transduce a target which can be in-vitro (e.g. an air-liquid interface culture) or in-vivo (e.g. a humanized mouse). Subsequently, the viral genomes are extracted and used to create a new batch of virus (2). Repeating this results in the enrichment of virus variants that are proficient in transducing the target cells (3). The same strategy can also be employed for the evolution of envelope proteins for enveloped viruses. (b) Retroviruses and lentiviruses have their receptor binding proteins (e.g. gp120 and gp41 in the case of HIV1) located on their viral envelope. Such receptor binding proteins can be replaced by envelope proteins to target a desired cell type (for instance the multi-tropic VSV-G or lung-tropic F/HN), via a process called pseudotyping.
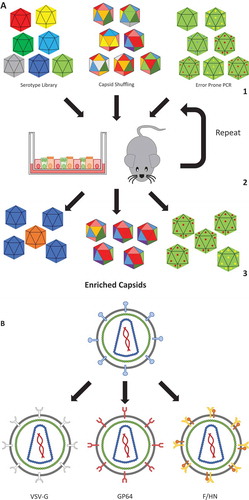
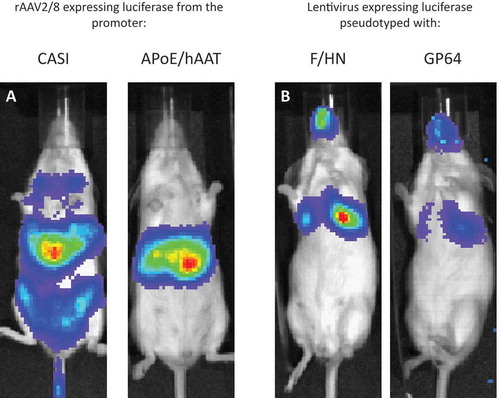
Figure 3. Pseudotype and promoter choice can influence transgene expression.
(a) Mice were administered with a similar dose (1e11 genome copies) of rAAV2/8 injected intravenously and imaged 7 days post-dosing. The only difference is the choice of promoter to drive luciferase reporter expression. CASI is a ubiquitous promoter and shows that rAAV8 transduces the liver but also other parts of the mouse. Replacing CASI with the liver-specific APoE/hAAT promoter maintains strong expression in the liver and largely prevents expression in off-target cells. (b) Mice were administered with a similar dose (1e8 transducing units) of the same rLV vector configuration expressing luciferase reporter gene, but pseudotyped with either F/HN or GP64, and were live imaged 7 days post-dosing. Increased transduction efficiency can be explained in part by the distribution of receptors. F/HN has direct access to its receptors (sialic acid) on the apical surface of the epithelium. Binding to GP64 (and VSV-G) receptors requires pre-treatment (1% methylcellulose used here) that may help access receptors on the basolateral membrane of the polarized epithelium (see )).