Figures & data
Figure 2. Flow chart of the main manufacturing steps of biotherapeutic particles – cell culture, purification and quality control, and its final usage in the different field of gene therapy, vaccination, oncolytic therapy and tumor vaccines. During the manufacturing platform, the main goals are to reduce the process time and cost as well as increase the recovery yields and product quality.
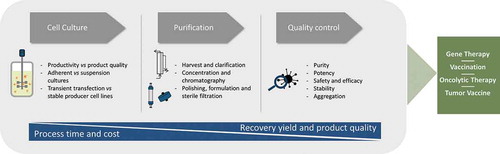
Figure 3. Effect of the different upstream processing parameters and culture options in the downstream processing.

Figure 4. Comparison of traditional configuration batch TFF with single-pass TFF, where the recirculation loop is eliminated.
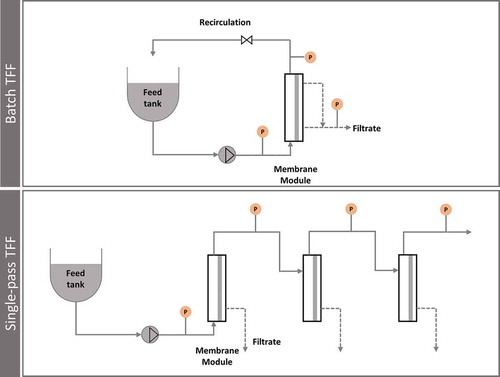
Table 1. Examples of recent purification processes for different biotherapeutic particles. The production system, as well as the purification steps and the recovery yields, are described.
Table 2. Specific analytical assays used in virus-based therapeutics and protein therapeutics (mAbs) for final product, categorized by identity, potency, quantity, purity and safety.
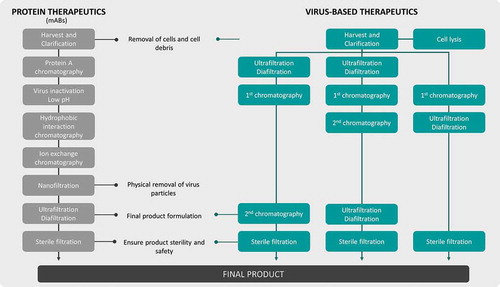
Figure 5. Schematic comparison of a common mAbs purification process vs virus-based therapeutics purification alternatives. MAbs purification processes are already well established in opposition to virus-based particles purification, where several combinations and different techniques (e.g. chromatography operations) can be used. Sterile filtration step cannot be performed for particles larger than 0.2µm.