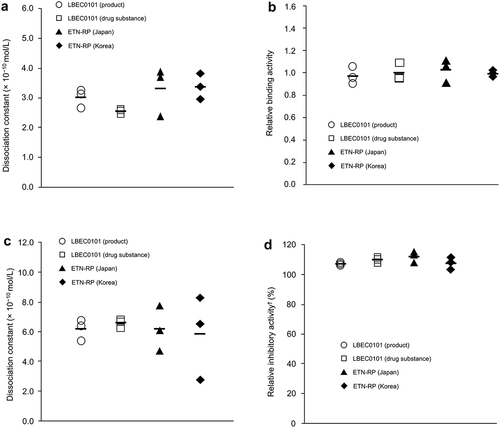
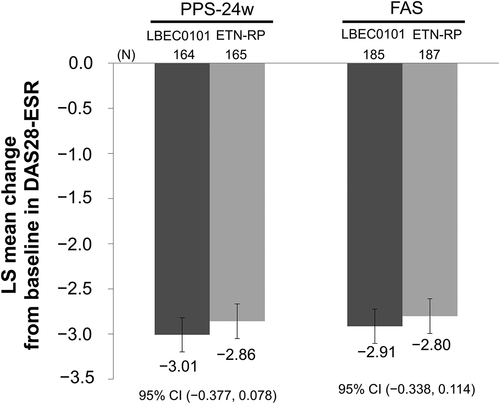
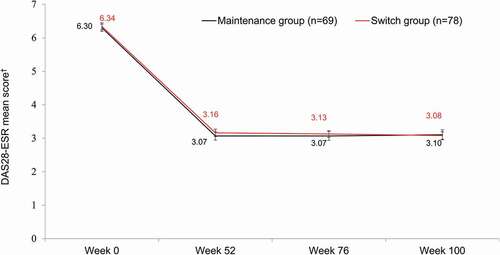
Figures & data
Table 1. Summary of pharmacokinetic parameters of LBEC0101 and etanercept reference product after a single 25-mg subcutaneous injection in healthy males
Table 2. Summary of AEs during the Phase III double-blind study and the extension study