Figures & data
Table 1. Demographic characteristics of the 151 patients included in the analysis.
Figure 1. PASI improvement after initiation of secukinumab therapy, which is maintained throughout the 136-week observation period.
LOCF: last observation carried forward imputation; PASI: Psoriasis Area and Severity Index; w: week; y: year. (a) Changes in PASI 75, PASI 90, and PASI 100 during 136 weeks of treatment. Data are presented as percentages.(b) Changes in absolute PASI scores over 136 weeks of treatment. Average PASI values are reported for each time point. The sidebox reports the percentage of patients who reached that particular time point at the moment of the analysis.
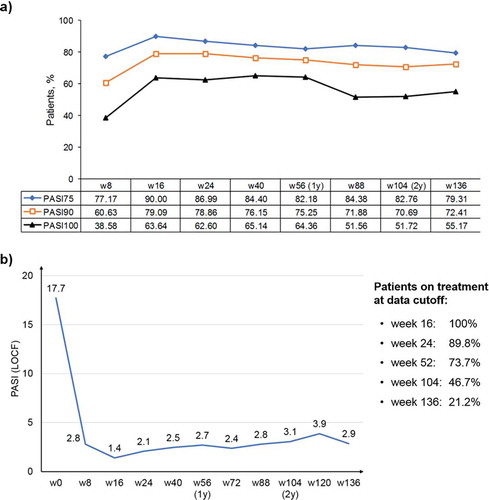
Table 2. Univariate logistic regression analysis of variables associated with PASI75 response.
Table 3. Univariate logistic regression analysis of variables associated with PASI90 response.
Table 4. Univariate logistic regression analysis of variables associated with PASI100 response.
Table 5. Multivariate logistic regression analysis (stepwise analysis) of predictors of PASI response after treatment with secukinumab at 1 and 2 years.
Figure 2. Patient with Down's syndrome prior to initiating secukinumab and after 104 weeks of therapy.
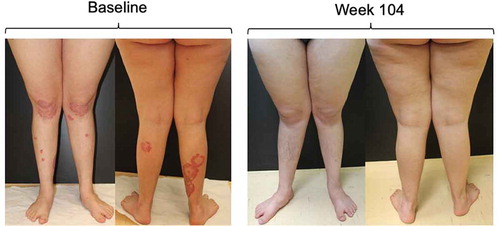
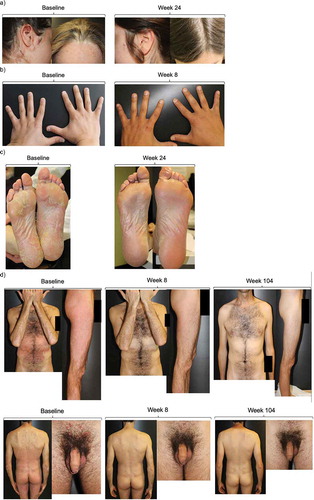
Figure 3. Plaque psoriasis in difficult-to-treat locations before and after initiation of secukinumab therapy.
(a) Patient with head and scalp plaques prior to initiating secukinumab and after 24 weeks of therapy. (b) Patient with nail psoriasis prior to initiating secukinumab and after 8 weeks of therapy. (c) Patient with plantar plaques prior to initiating secukinumab and after 24 weeks of therapy. (d) Patient with torso, leg, and, genital localizations at baseline and after 8 and 104 weeks of treatment.