Figures & data
Table 1. Baseline clinical and serological characteristics of patients.
Figure 1. Evaluation of joint count and inflammatory markers during follow-up in patients with PsA and AS pooled together. T0: 169 patients, T6: 145 patients, and T12: 129 patients. The Wilcoxon test was used to compare matched values (T0, T6, and T12).
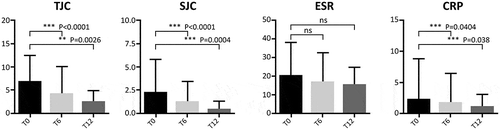
Figure 2. Evaluation of DAPSA during follow-up in patients with PsA. T0: 130 patients, T6: 109 patients, and T12: 99 patients. The Wilcoxon test was used to compare paired disease activity scores at different time points (T0, T6, and T12).
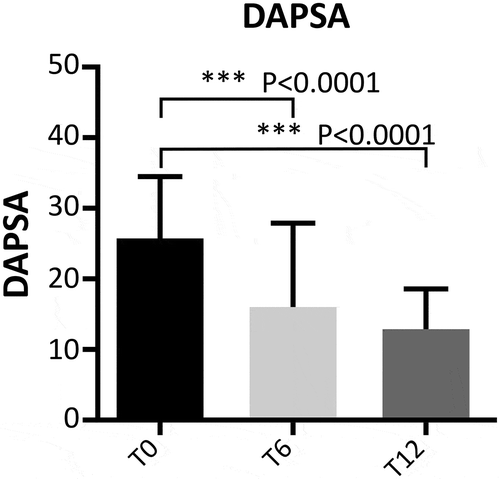
Figure 3. Evaluation of BASDAI (Panel A) and ASDAS-CRP (Panel B) during follow-up. BASDAI Bath Ankylosing Spondylitis Disease Activity Index, ASDAS-CRP Ankylosing Spondylitis Disease Activity Score with C-reactive protein. T0: 169 patients, T6: 145 patients, and T12: 129 patients. The paired scores at T0, T6, and T12 were compared using the Wilcoxon test in patient with PsA and AS, pooled together.
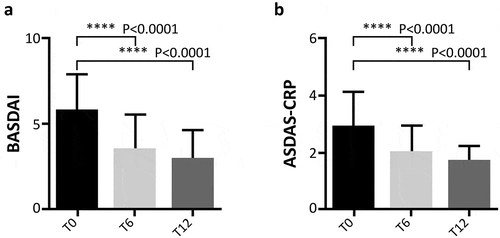
Figure 4. Twelve months retention rate of secukinumab in AS and PsA patients according to gender. Panel A. Global retention rate (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel B. Global retention rate according to gender (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel C. Retention Rate according to gender in AS patients T0: 39 patients, T6: 36 patients, and T12: 30 patients.; Panel D. Retention Rate according to gender in PsA patients (T0: 130 patients, T6: 109 patients, and T12: 99 patients).
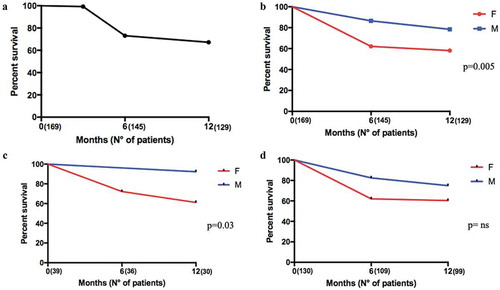
Figure 5. Twelve months retention rate of secukinumab in AS and PsA patients according to BMI and to line of biologics. Panel A. Retention Rate according to BMI (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel B. Retention Rate according to lines of biologics (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel C. Retention Rate according to the presence or absence of Methotrexate (T0: 169 patients, T6: 145 patients, and T12: 129 patients).
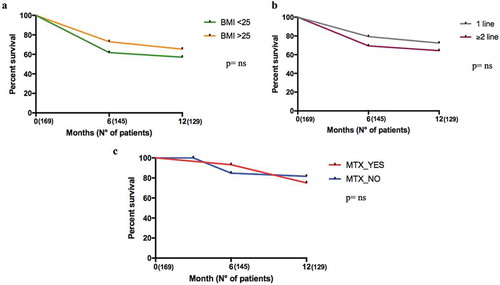
Table 2. Causes of treatment discontinuation in the study population.
