Figures & data
Figure 1. Psoriasis inflammatory cascade. Abbreviations: TNF, tumor necrosis factor; IL, interleukin; Th, T helper; IFN-γ, interferon gamma; γδ T-cells, gamma delta T cells. Elaborated from [Citation14]
![Figure 1. Psoriasis inflammatory cascade. Abbreviations: TNF, tumor necrosis factor; IL, interleukin; Th, T helper; IFN-γ, interferon gamma; γδ T-cells, gamma delta T cells. Elaborated from [Citation14]](/cms/asset/bfca9dee-5128-4829-8348-d44430bbb7ce/iebt_a_1849131_f0001_oc.jpg)
Figure 2. QoL improvement at week 12 in FIXTURE, ERASURE, and CLEAR. Data assessed with non-responder imputation
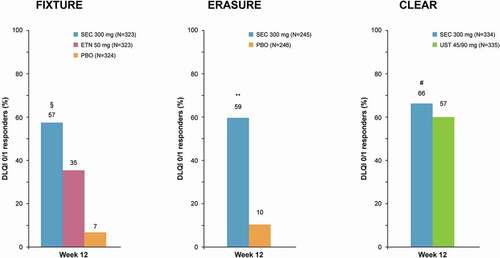
Table 1. ERASURE: Results from psoriasis symptoms diary at week 12
Table 2. FIXTURE: Results from psoriasis symptoms diary at week 12
Figure 3. QoL improvement at 1-year follow-up with EQ-5D-3 L in CLEAR. Data assessed with non-responder imputation. Ustekinumab dose: 45 mg for patients weighing ≤100 kg at baseline or 90 mg for patient weighing >100 kg. QoL, quality of life; SEC, secukinumab; UST, ustekinumab
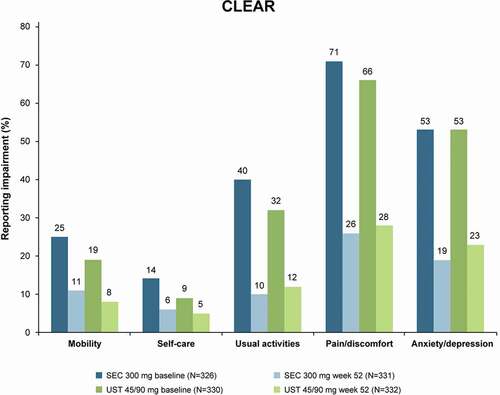
Figure 4. Changes in DLQI (A) and Family DLQI (B) after treatment with secukinumab in patients with psoriasis stratified by prior treatment history. Adapted from [Citation52] with permission
![Figure 4. Changes in DLQI (A) and Family DLQI (B) after treatment with secukinumab in patients with psoriasis stratified by prior treatment history. Adapted from [Citation52] with permission](/cms/asset/6d511b62-b571-4d6b-b880-d0020db563c7/iebt_a_1849131_f0004_oc.jpg)
Table 3. CORRONA Psoriasis Registry: changes in patient-reported outcomes at 6 months
