Figures & data
Figure 1. Toca 511 design and mechanism of action: (a) Toca 511 is a retroviral replicating vector (RRV) based on an amphotropic mouse gamma-retrovirus which also encodes an optimized yeast cytosine deaminase (CD) and preferentially infects tumors (LTR: long terminal repeat sequences, gag/pol/env: retroviral structural genes, IRES-CD: transgene expression cassette consisting of internal ribosome entry site linked to CD gene). (b) Upon 5-FC administration, optimized CD metabolizes the antifungal prodrug Toca FC (a proprietary formulation of 5-FC) to the active anticancer drug 5-FU directly within infected cancer cells. (c) Illustration of mechanism of action of Toca 511 and Toca FC causing antitumor immune activation via the tumor microenvironment: (1) Gamma-retrovirus Toca 511 selectively infects tumor, persists, and further spreads through tumor while delivering the CD gene; (2) Toca FC prodrug locally converts to 5-FU chemotherapeutic drug within the tumor, killing cancer cells and resulting in activation of antigen-presenting cells and T cell priming; (3) ‘Bystander effect’ of locally generated intratumoral 5-FU also eliminates adjacent immunosuppressive myeloid cells (myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs)), thereby activating antitumor immunity
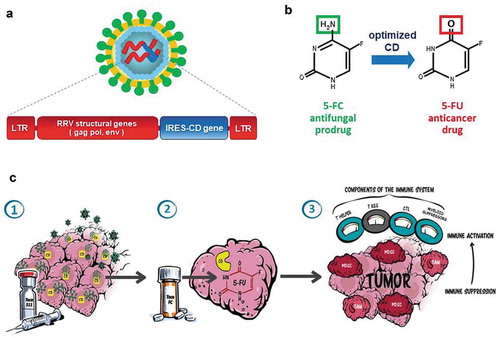
Table 1. Survival results from Phase 1 trials of Toca 511/Toca FC therapy in recurrent high-grade glioma patients (www.clinicaltrials.gov: NCT01156584, NCT01470794, NCT01985256)
Table 2. Objective Response, durableresponse and clinical benefit rates in the Phase 1 resection trial (NCT01470794)
Figure 2. ‘Swim lane’ representation of data from the Phase 1 resection study in recurrent high-grade glioma patients, higher dose cohorts (‘Phase 3 eligible’): Toca 511 and Toca FC leads to 26% long-term survival and 22% durable complete response (CR) rate. All responses are durable CRs and associated with long-term survival (see Cloughesy et al. Citation40)
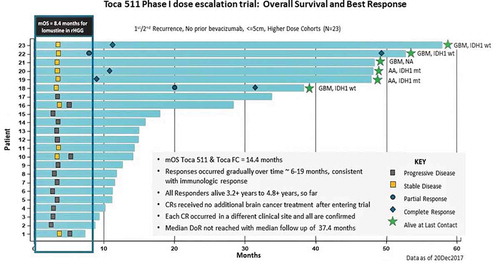
Figure 3. ‘TOCA 5’ Phase 2/3 clinical trial of Toca 511/Toca FC for recurrent high-grade glioma (HGG). (a) Study design of TOCA 5 trial. This was the largest randomized study conducted in the setting of recurrent HGG. GBM: glioblastoma multiforme, AA: anaplastic astrocytoma, IDH1: isocitrate dehydrogenase-1, KPS: Karnofsky Performance Score, SOC: standard of care. (b) Kaplan–Meier plot of overall survival by time from last Temozolomide treatment to randomization (ITT population). (c) Kaplan–Meier plot of overall survival by number of Toca FC cycles in the Phase 2/3 trial (intention-to-treat (ITT) population)
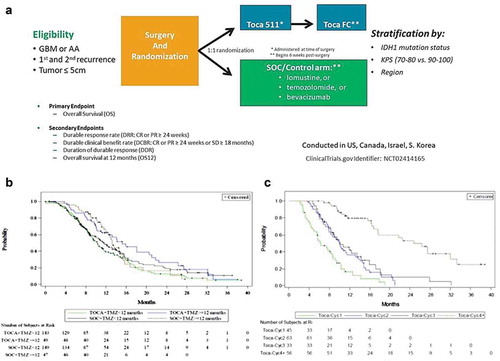
Table 3. No major safety signals with Toca 511 and Toca FC in Phase 3 trial
Figure 4. Overall survival in patient subgroup with two or more recurrences (intention-to-treat population). Left panel: Kaplan–Meier plot of overall survival in the second recurrence subgroup; Toca 511 and Toca FC showed 57% reduction in risk of death (~2X median survival over standard of care (SOC)). HR: hazard ratio, OS: overall survival. Right panel: Key parameters of the Toca 511/Toca FC treatment vs. SOC groups, showing good comparability. KPS: Karnofsky Performance Score, IDH: isocitrate dehydrogenase
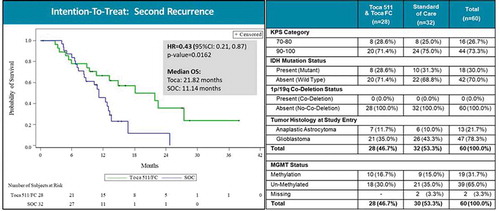
Figure 5. Overall survival by tumor histology in patient subgroup with two or more recurrences (intention-to-treat population). Left panel: Kaplan–Meier plot of overall survival in second recurrence subgroup patients with glioblastoma multiforme (GBM). Right panel: Kaplan–Meier plot of overall survival in second recurrence subgroup patients with anaplastic astrocytoma (AA). HR: hazard ratio, OS: overall survival
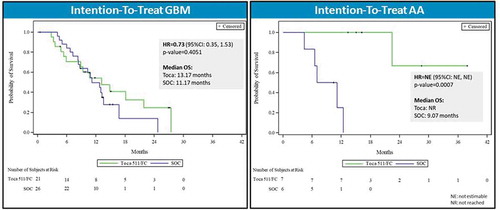
Table 4. Median number of drug treatments for the intention-to-treat group (Toca FC) and the standard-of-care (SOC) control arm in the Phase 3 trial
