Figures & data
Table 1. Patient demographics and baseline characteristics.
Figure 1. Study design and outcomes. a) Inclusion and exclusion criteria for patients included in the outcome analyses of this study. All patients were enrolled in the real-world AIMS study and in keeping with the inclusion criteria of the parent study were ≥18 years of age, had a clinical diagnosis of RA and MSRC test results. b) Mean change from baseline in absolute CDAI at 12- and 24-week follow-up visits for predicted non-responders to TNFi therapy who were treated with a TNFi (PNR-TNFi) or a b/tsDMARD with an alternative mechanism of action (PNR-altMOA).
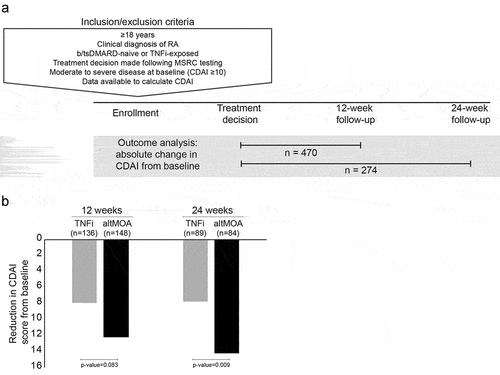
Table 2. Mean change in CDAI scores from baseline to 12-week and 24-week follow-up visits in the AIMS cohort subsets.
