Figures & data
Table 1. New myasthenia gravis therapies approved and under study.
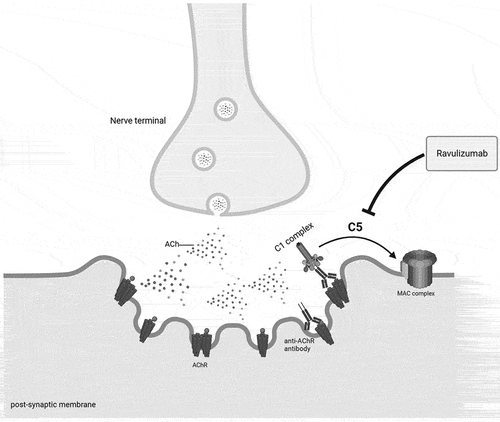
Figure 1. Pathophysiology of complement activation in MG: anti-acetylcholine (Ach) receptor (AChR) antibodies activate the complement cascade through the classical pathway by binding C1q con the Fc domain. The subsequent formation of C5 convertase initiates the terminal pathway which ultimately leads to the formation of the membrane attack complex (MAC), a lytic pore on the postsynaptic membrane. The final effect is a focal lysis of the neuromuscular junction (NMJ), with disruption of the postsynaptic folds and loss of AChRs. Ravulizumab binds with high affinity to C5, inhibiting its enzymatic cleavage and thereby preventing the MAC formation. Created with Biorender.com.