Figures & data
Table 1. Demographic and clinical characteristics of the cohort (122 patients).
Figure 1. Effect of guselkumab on Psoriasis Area and Severity Index (PASI) score and achievement of PASI 75, 90, and 100 responses over 148 weeks in patients with moderate-to-severe psoriasis. Missing values for intermediate visits were imputed with the last observation carried forward (LOCF) method. a: mean PASI scores in the overall population. B: improvement in PASI 75, 90, and 100 responses in the cohort of all patients. C: mean PASI scores in obese (BMI≥30) and non-obese (BMI<30) patients. Improvement in PASI 75 (D), 90 (E), and 100 (F) responses in obese and non-obese patients.
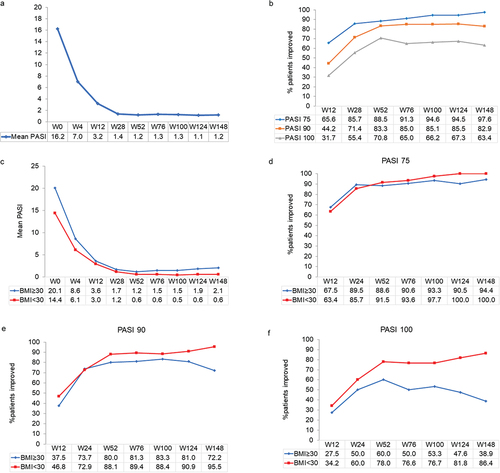
Figure 2. Effect of guselkumab on PASI 75, 90, and 100 responses over 148 weeks in bio-naïve and bio-experienced patients with moderate-to-severe psoriasis (panels a, b and c), in bio-experienced patients with moderate-to-severe psoriasis who had received<3 or ≥ 3 previous lines of biologic therapy (PBT) (panels d, e and f), and in patients with moderate-to-severe psoriasis who switched from ustekinumab over 100 weeks (panel g). Missing values for intermediate visits were imputed with the last observation carried forward (LOCF) method.
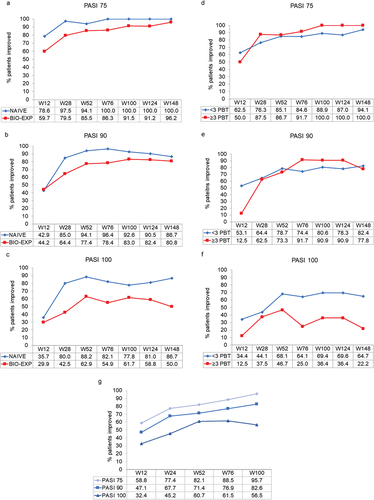
Table 2. Univariate logistic regression analysis of variables associated with PASI 75 response.
Table 3. Univariate logistic regression analysis of variables associated with PASI 90 response.
Table 4. Univariate logistic regression analysis of variables associated with PASI 100 response.
Table 5. Multivariate logistic regression analysis (stepwise analysis) of predictors of PASI 75, PASI 90, and PASI 100 response after treatment with guselkumab.
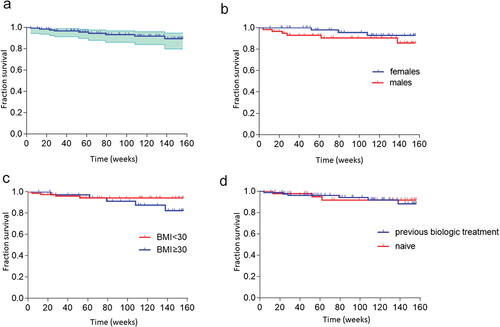
Figure 3. Drug survival rates for guselkumab monotherapy in the entire cohort (a) and grouped according to sex (b), BMI<30 or ≥30 kg/m2 (c), or prior treatment with a biologic (d).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.