Figures & data
Table 1. General demographic data and reported efficacy in adalimumab and infliximab biosimilar-treated patients.
Figure 1. Subjective efficacy of adalimumab and infliximab biosimilars in patients with inflammatory bowel disease.
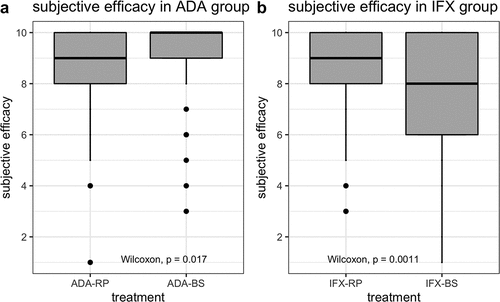
Table 2. Reported adverse events during infliximab and adalimumab biosimilar treatment.
