Figures & data
Figure 1. Participant disposition.
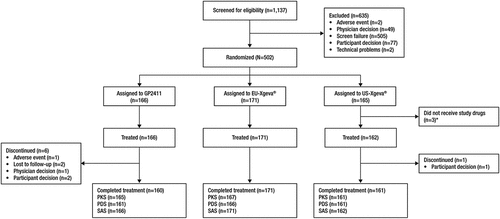
Table 1. Participant demographics and baseline characteristics.
Figure 2. Geometric mean ratios and 90% CI of primary PK parameters.
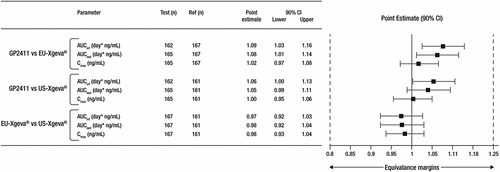
Figure 3. Mean drug serum concentration–time profiles. (a) Mean (SD) linear scale. (b) Mean, semi-logarithmic scale.
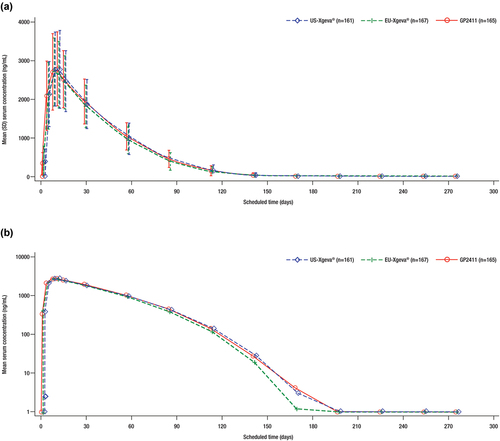
Table 2. Pharmacokinetic endpoints.
Figure 4. Geometric mean ratio and 95% CIs of AUEC of percentage change from baseline in serum CTX.
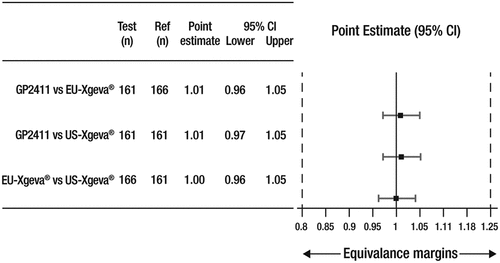
Figure 5. Change from baseline in PD endpoints. (a) serum CTX levels. (b) serum PINP levels.
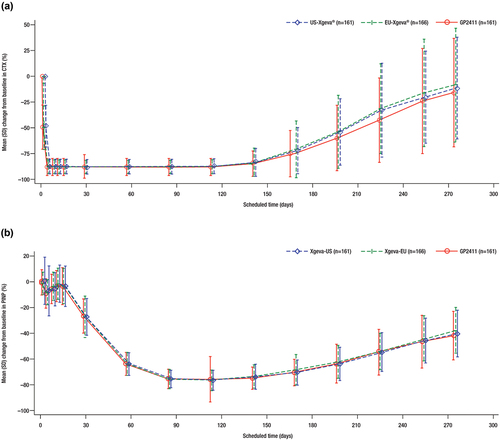
Table 3. Treatment-emergent adverse events reported in ≥2% of participants in any treatment group.
Table 4. Incidence of ADAs and immunogenicity outcomes.
Data availability statement
Data from this study are available from the corresponding author on request.
