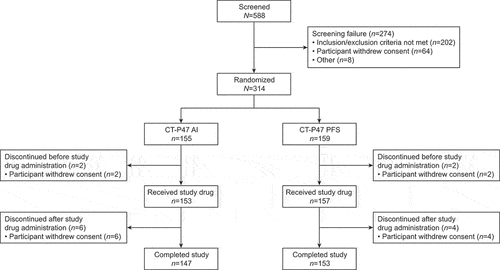
Figures & data
Table 1. Participant demographics and baseline characteristics (intent-to-treat).
Figure 2. Mean (SD) serum concentrations of CT-P47 for CT-P47 AI and CT-P47 PFS (PK set).
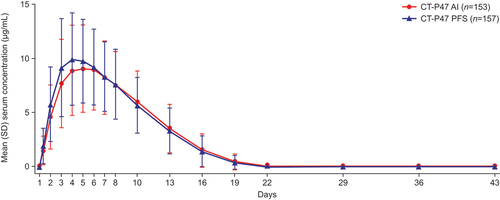
Figure 3. Summary and statistical analysis of the primary pharmacokinetic endpoints (PK set).
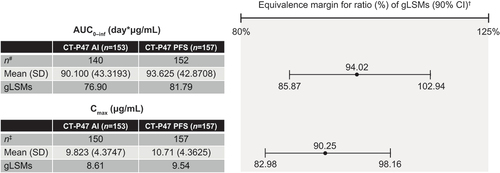
Table 2. Secondary pharmacokinetic results (PK set).
Table 3. Adverse events (safety set).
Supplemental Material
Download MS Word (250.5 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.