Figures & data
Table 1. Guideline recommendations for the use of infliximab across the approved indications.
Table 2. Approved indications for infliximab medicines, including biosimilars, in Europe and the USA.
Figure 1. Timeline highlighting key studies for GP1111. ACR20: American College of Rheumatology 20% improvement criteria; AxSpA: axial spondyloarthritis; CD: Crohn’s disease; EMA: European Medicines Agency; FDA: US Food and Drug Administration; HBI: Harvey–Bradshaw Index; IV: intravenous; OLE: open-label extension; PsA: psoriatic arthritis; PMS: Partial Mayo Score; RA: rheumatoid arthritis; ref-IFX: reference infliximab; UC, ulcerative colitis.
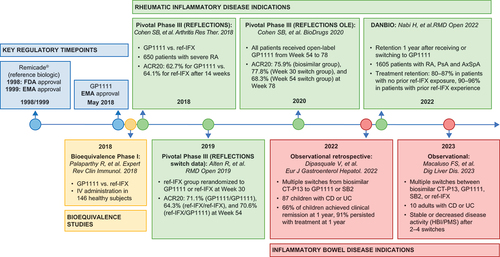
Figure 2. Timeline highlighting key studies for GP1111. Adapted from Cohen SB, et al. Arthritis Res Ther. 2018;20:155 [Citation37]. Image adapted under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.Org/licenses/by/4.0/).
![Figure 2. Timeline highlighting key studies for GP1111. Adapted from Cohen SB, et al. Arthritis Res Ther. 2018;20:155 [Citation37]. Image adapted under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.Org/licenses/by/4.0/).](/cms/asset/24b66dce-3311-4947-ab96-3fd4859dea95/iebt_a_2377298_f0002_oc.jpg)
