Figures & data
Figure 1. PRISMA diagram.
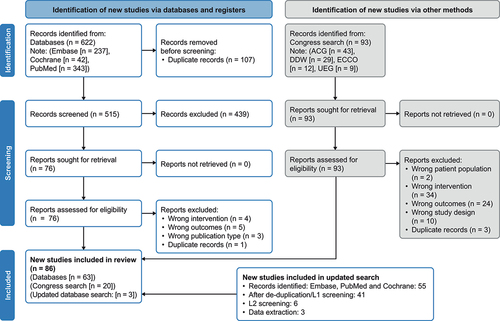
Table 1. RCTs evaluating switching from IFX originator (oIFX) to biosimilar (N = 2), IFX biosimilar to oIFX (N = 1) or IFX biosimilar maintenance (N = 1).
Table 2. Mucosal healing associated with switching from (A) IFX originator (oIFX) to biosimilar (N = 4) or (B) IFX biosimilar to oIFX or biosimilar maintenance (N = 1) in patients with CD in RCTs and longitudinal studies.
Table 3. Summary of TEAEs in RCTs, and overall AEs and SAEs in longitudinal studies in patients with IBD switching from IFX originator (oIFX) to biosimilar.
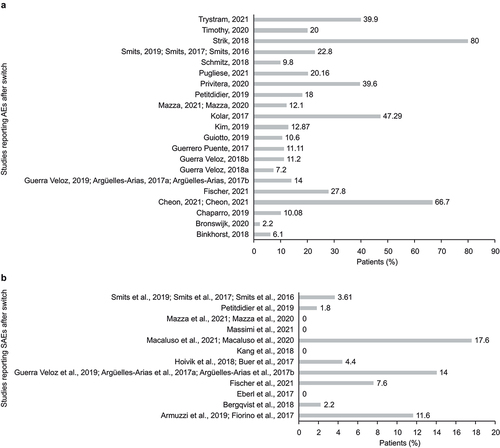
Figure 3. Percentage of patients with (a) AEs and (b) SAEs in patients with IBD after switching from oIFX to biosimilar in longitudinal studies.
Supplemental Material
Download MS Word (154.9 KB)Data availability statement
All data generated or analyzed during this study are included in this published article or as supplementary information files.