Figures & data
Table 1. Cell therapy clinical trials.
Table 2. Gene therapy clinical trials.
Figure 1. RPGRORF15 is located in the connecting cilia of rods and cones. Its proposed function is to enable the transport of different molecules along the connecting cilia, for which its glutamylation and its interaction with other proteins seem to be essential.
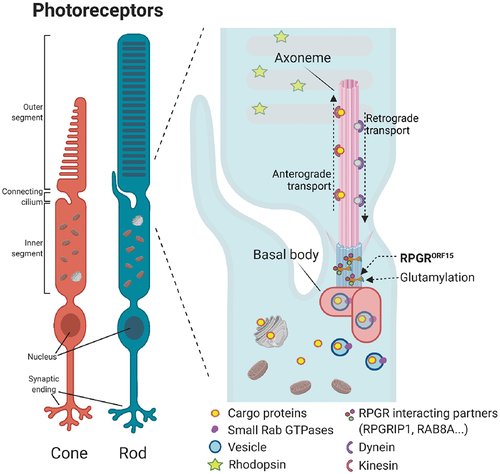
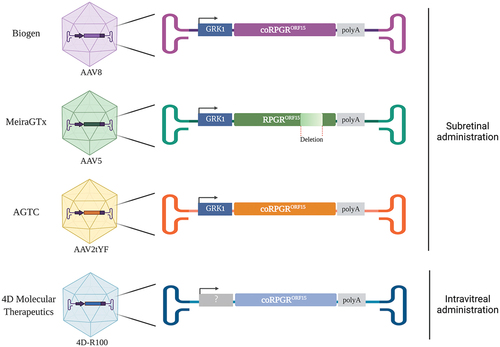
Figure 2. Recombinant AAV.RPGR vectors currently being tested in human clinical trials. The three AAV vectors administered in the subretinal space use the human GRK1 promoter to drive the expression of RPGRORF15. The vectors developed by Biogen Inc., AGTC and 4D Molecular Therapeutics contain a codon-optimized (co) version of the human RPGRORF15 gene whilst the vector developed by MeiraGTx contains a shortened version of the human RPGRORF15. The AAV serotype capsids used to develop these vectors are AAV8 (Biogen), AAV5 (MeiraGTx), an AAV2 variant, with three tyrosine to phenylalanine mutations (AAV2tYF) (AGTC), and an AAV capsid variant named 4D-R100 suitable for intravitreal administration (4D Molecular Therapeutics).