Figures & data
Figure 1. The CD47-SIRPα signaling for regulation of phagocytes. (a) CD47 is a transmembrane protein with an immunoglobulin (Ig)-V–like extracellular domain, five putative membrane-spanning segments, and a short cytoplasmic tail. Signal regulatory protein α (SIRPα) consists of an N-terminus Ig-V–like domain and two Ig-C–domains in its extracellular region as well as two immunoreceptor tyrosine-based inhibition motifs (ITIMs) in the cytoplasmic region. The tyrosine-phosphorylated ITIMs of SIRPα bind and thereby activate the protein tyrosine phosphatase Shp1. Ligation of SIRPα on macrophages by CD47 on antibody-opsonized red blood cells (RBC) promotes tyrosine phosphorylation of SIRPα and its subsequent association with Shp1, which in turn inhibits RBC phagocytosis by macrophages induced by the interaction of RBC-bound antibodies with the Fcγ receptor (FcγR) on macrophages. (b) The CD47-SIRPα interaction between tumor cells and macrophages negatively regulates macrophage-mediated phagocytosis of tumor cells, contributing to tumor evasion of immunosurveillance
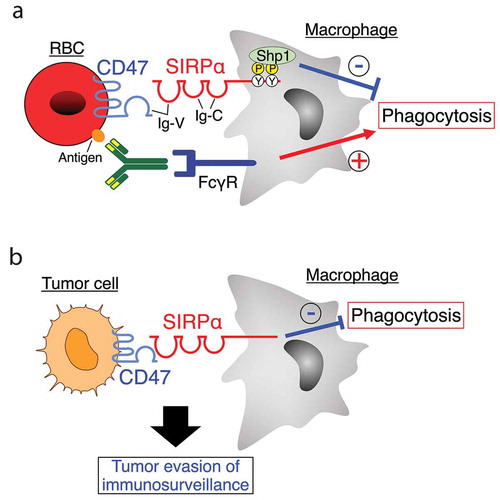
Figure 2. Blockade of CD47 or SIRPα promotes tumor killing by professional phagocytes. The phagocytosis of target tumor cells by professional phagocytes, such as macrophages, is regulated by activating and inhibitory signals, specifically via the interaction of receptors and their ligands. Calreticulin (CRT) and signaling lymphocytic activation molecule family 7 (SLAMF7) on tumor cells have been demonstrated to serve as an activating signal by interacting with low-density lipoprotein receptor-related protein 1 (LRP-1) and SLAMF7 on macrophages, respectively [Citation18,Citation19]. When bound by antibodies, antigens (tumor-associated antigens) on tumor cells are recognized by the Fcγ receptor (FcγR) on macrophages and initiate activating signals. Blockade of CD47 or signal regulatory protein α (SIRPα) by agents such as antibodies disrupts the inhibitory signal mediated by the interaction of CD47 on tumor cells with SIRPα on macrophages, which in turn promotes phagocytosis induced by activating signals. Ab, antibody
![Figure 2. Blockade of CD47 or SIRPα promotes tumor killing by professional phagocytes. The phagocytosis of target tumor cells by professional phagocytes, such as macrophages, is regulated by activating and inhibitory signals, specifically via the interaction of receptors and their ligands. Calreticulin (CRT) and signaling lymphocytic activation molecule family 7 (SLAMF7) on tumor cells have been demonstrated to serve as an activating signal by interacting with low-density lipoprotein receptor-related protein 1 (LRP-1) and SLAMF7 on macrophages, respectively [Citation18,Citation19]. When bound by antibodies, antigens (tumor-associated antigens) on tumor cells are recognized by the Fcγ receptor (FcγR) on macrophages and initiate activating signals. Blockade of CD47 or signal regulatory protein α (SIRPα) by agents such as antibodies disrupts the inhibitory signal mediated by the interaction of CD47 on tumor cells with SIRPα on macrophages, which in turn promotes phagocytosis induced by activating signals. Ab, antibody](/cms/asset/61827c7b-b17c-467c-b103-a596ccccbc3d/iett_a_1811855_f0002_oc.jpg)
Figure 3. Antitumor effect of CD47 or SIRPα blockade involves both innate and adaptive immune responses. Blockade of the interaction of signal regulatory protein α (SIRPα) on macrophages (MΦ) or dendritic cells (DCs) with CD47 on tumor cells using CD47- or SIRPα-targeting agents, such as anti-CD47 or anti-SIRPα antibodies (Abs), promotes the phagocytosis of tumor cells and tumor cell-derived substances by these antigen-presenting cells as well as the consequent priming of tumor-specific cytotoxic T cells [Citation20,Citation21]. Disruption by antibodies to CD47 or SIRPα of the CD47-SIRPα interaction between neutrophils and tumor cells also enhances the neutrophil-mediated killing of antibody-opsonized tumor cells [Citation10]. Moreover, blocking CD47 on tumor-specific cytotoxic T cells and natural killer (NK) cells with anti-CD47 antibodies increases their cellular cytotoxicity against tumor cells [Citation25,Citation28]
![Figure 3. Antitumor effect of CD47 or SIRPα blockade involves both innate and adaptive immune responses. Blockade of the interaction of signal regulatory protein α (SIRPα) on macrophages (MΦ) or dendritic cells (DCs) with CD47 on tumor cells using CD47- or SIRPα-targeting agents, such as anti-CD47 or anti-SIRPα antibodies (Abs), promotes the phagocytosis of tumor cells and tumor cell-derived substances by these antigen-presenting cells as well as the consequent priming of tumor-specific cytotoxic T cells [Citation20,Citation21]. Disruption by antibodies to CD47 or SIRPα of the CD47-SIRPα interaction between neutrophils and tumor cells also enhances the neutrophil-mediated killing of antibody-opsonized tumor cells [Citation10]. Moreover, blocking CD47 on tumor-specific cytotoxic T cells and natural killer (NK) cells with anti-CD47 antibodies increases their cellular cytotoxicity against tumor cells [Citation25,Citation28]](/cms/asset/7de3fe71-ab61-44c1-b56b-ab23ebe35886/iett_a_1811855_f0003_oc.jpg)
