Figures & data
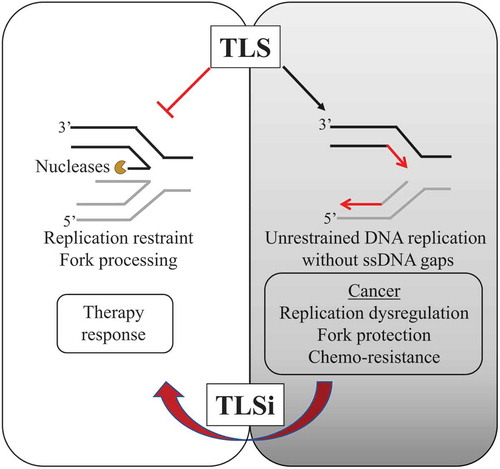
Figure 1. Schematic illustration to depict replication fork dynamics upon RS
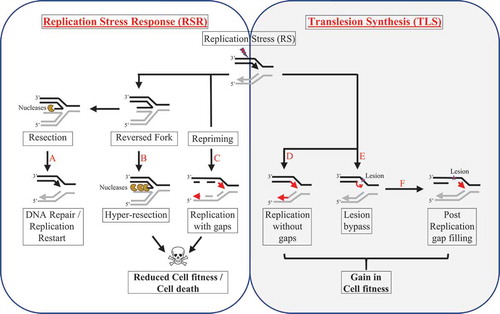
Figure 2. The role of oncogene-induced RS in the development of cancer
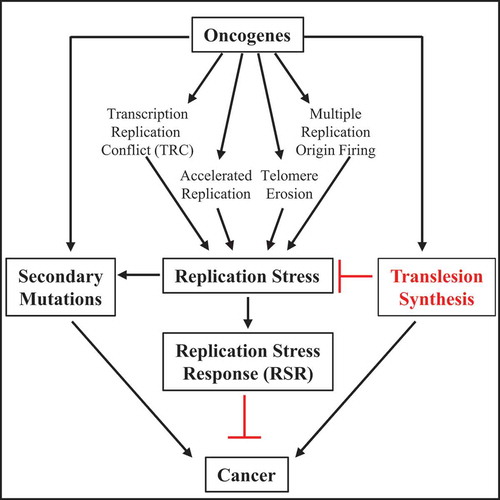
Figure 3. Schematic illustration to depict TLS-induced chemo-resistance in cancer
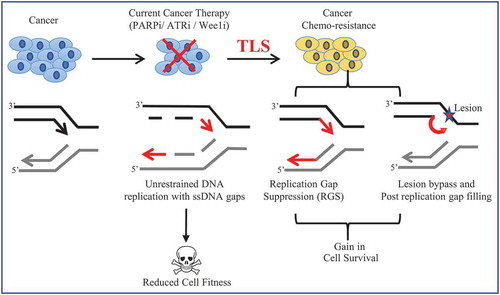
Figure 4. Model proposing TLS as a new evolving target for cancer therapy