Figures & data
Figure 1. Cartoon illustrating the sequential steps involved in the uptake of long-chain fatty acids by cells. 1. Release of fatty acids from (interstitial) albumin; 2. Binding in the hydrophobic cavity of CD36 which can accommodate up to two fatty acids at a time; 3. Guidance of the fatty acid through the CD36 ectodomain interior to pass the unstirred water layer and be exposed to the plasma membrane surface; 4. Exit of the fatty acid from CD36 to the outer leaflet of the phospholipid bilayer; 5. Transmembrane translocation (‘flip-flop’) of single fatty acids; 6. Desorption of fatty acids from the inner leaflet of the phospholipid bilayer and binding to the interior of FABP that is anchored by binding to the intracellular part of CD36; 7. Diffusion into the soluble cytoplasm of the fatty acid–FABP complex toward sites of intracellular fatty acid metabolism. Note that proteins and membranes, and their putative mutual interactions are not drawn to scale. Reproduced with permission from Glatz JFC, Luiken JJFP. Time for a détente in the war on the mechanism of cellular fatty acid uptake. J Lipid Res. 2020;61:1300–1303 [Citation14]
![Figure 1. Cartoon illustrating the sequential steps involved in the uptake of long-chain fatty acids by cells. 1. Release of fatty acids from (interstitial) albumin; 2. Binding in the hydrophobic cavity of CD36 which can accommodate up to two fatty acids at a time; 3. Guidance of the fatty acid through the CD36 ectodomain interior to pass the unstirred water layer and be exposed to the plasma membrane surface; 4. Exit of the fatty acid from CD36 to the outer leaflet of the phospholipid bilayer; 5. Transmembrane translocation (‘flip-flop’) of single fatty acids; 6. Desorption of fatty acids from the inner leaflet of the phospholipid bilayer and binding to the interior of FABP that is anchored by binding to the intracellular part of CD36; 7. Diffusion into the soluble cytoplasm of the fatty acid–FABP complex toward sites of intracellular fatty acid metabolism. Note that proteins and membranes, and their putative mutual interactions are not drawn to scale. Reproduced with permission from Glatz JFC, Luiken JJFP. Time for a détente in the war on the mechanism of cellular fatty acid uptake. J Lipid Res. 2020;61:1300–1303 [Citation14]](/cms/asset/2f082b23-a44e-4a73-9660-5add74d17608/iett_a_1941865_f0001_oc.jpg)
Figure 2. Schematic presentation of both the facilitatory and regulatory roles of CD36 in (long-chain) fatty acid uptake into cardiomyocytes. Middle part of figure: At sarcolemmal lipid rafts, CD36, presumably in interaction with the peripheral membrane protein FABPpm and, at the intracellular site, with cytoplasmic FABP (FABPc), facilitates the entry of fatty acids into the cell. Left part of figure: Short-term regulation (i.e. minutes) of the rate of cellular fatty acid uptake occurs by reversible intracellular recycling (by vesicular transport) of CD36 from an endosomal storage compartment to the sarcolemma, which is triggered by changes in the frequency of muscle contraction or by plasma insulin. These latter triggers are mediated by the AMPK-activated and insulin signaling cascades, respectively, which converge at AS160. Right part of figure: Long-term regulation of cellular fatty acid uptake occurs via changes in CD36 gene transcription, mediated amongst others by fatty acid-induced PPAR activation, HIF-1, and C/EBP. Reproduced with permission from Glatz JFC, Luiken JJFP. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–26 [Citation20]
![Figure 2. Schematic presentation of both the facilitatory and regulatory roles of CD36 in (long-chain) fatty acid uptake into cardiomyocytes. Middle part of figure: At sarcolemmal lipid rafts, CD36, presumably in interaction with the peripheral membrane protein FABPpm and, at the intracellular site, with cytoplasmic FABP (FABPc), facilitates the entry of fatty acids into the cell. Left part of figure: Short-term regulation (i.e. minutes) of the rate of cellular fatty acid uptake occurs by reversible intracellular recycling (by vesicular transport) of CD36 from an endosomal storage compartment to the sarcolemma, which is triggered by changes in the frequency of muscle contraction or by plasma insulin. These latter triggers are mediated by the AMPK-activated and insulin signaling cascades, respectively, which converge at AS160. Right part of figure: Long-term regulation of cellular fatty acid uptake occurs via changes in CD36 gene transcription, mediated amongst others by fatty acid-induced PPAR activation, HIF-1, and C/EBP. Reproduced with permission from Glatz JFC, Luiken JJFP. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–26 [Citation20]](/cms/asset/442c4e52-34a4-4216-9756-45028b7b955f/iett_a_1941865_f0002_oc.jpg)
Figure 3. Illustration of the four distinct levels at which the functioning of CD36 in cardiomyocyte fatty acid uptake can be modulated. 1. Intervention of signaling pathways affecting CD36 gene expression; 2. Intervention in co-translational modification of CD36, in particular its glycosylation; 3. Modulation of reversible vesicular transport of CD36 between an endosomal storage compartment and the sarcolemma (subcellular recycling); 4. Influencing CD36 palmitoylation, its insertion into the sarcolemma, and putative activation (e.g. interaction with other membrane-associated proteins)
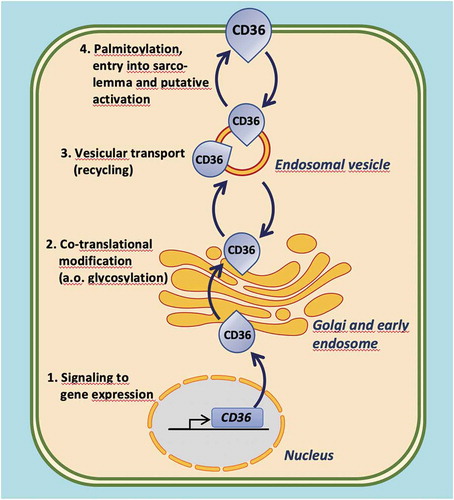
Table 1. Compounds affecting CD36 function or gene expression and their reported effects
